- Article
- Source: Campus Sanofi
- 30 Jun 2024
An Overview of the impact of Type 2 Inflammation in COPD: Insights from Dr. Surya Bhatt

Understanding COPD and Inflammation Pathways
COPD has historically been viewed through the lens of neutrophilic inflammation, driven primarily by the DH1 and DH17 pathways. However, Dr. Bhatt highlights a shift in understanding, noting that "there's increasing recognition that Type 2 inflammation may actually play a role in COPD." This inflammation is not as straightforward to detect, with varying degrees of presence in the patient population. "It's between 20 to 40 percent of patients seem to have some evidence of Type 2 inflammation," Dr. Bhatt explains.
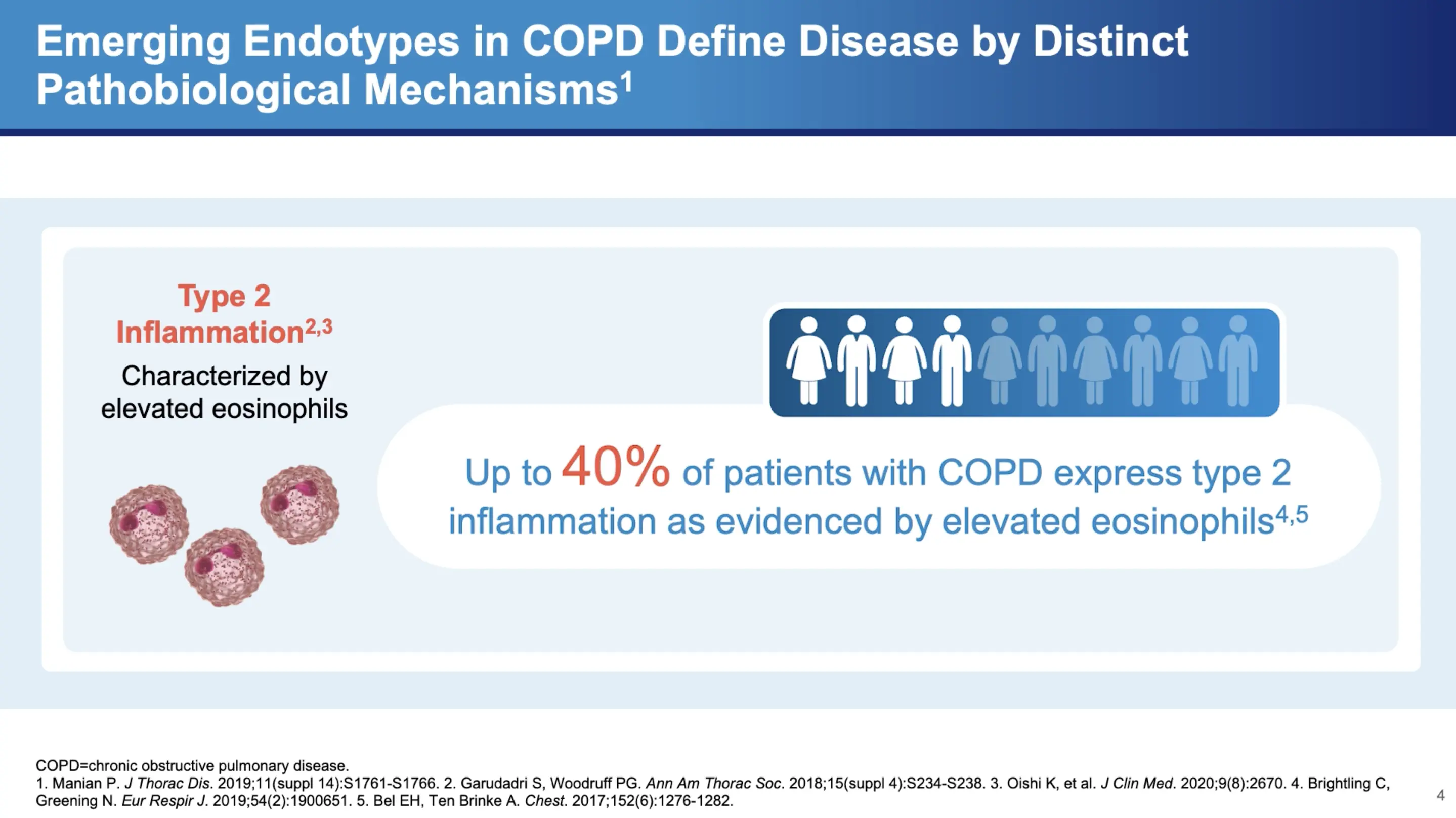
Diagnostic Markers of Type 2 Inflammation
One of the key markers for detecting Type 2 inflammation is the eosinophil count in the blood. Dr. Bhatt points out the diagnostic value of this marker: "It correlates relatively well with eosinophilic inflammation in the lungs and it's more likely to be specific than sensitive." This means that a high eosinophil count can be a reliable indicator of Type 2 inflammatory activity within the lungs. However, he also cautions that fluctuating eosinophil levels can complicate the diagnosis, stating, "if you don't find it, that doesn't mean they don't have Type 2 inflammation."
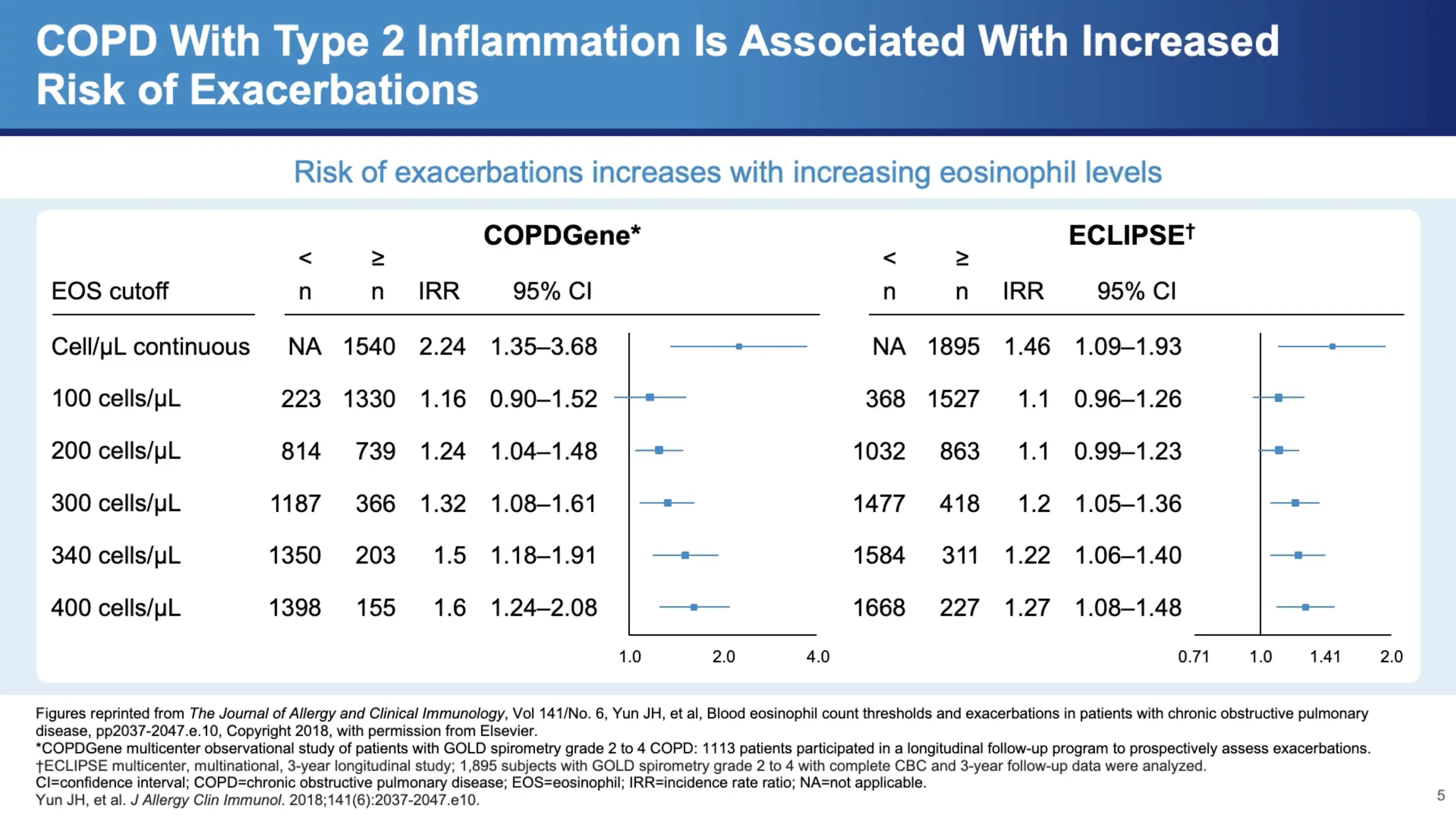
Clinical Implications of Type 2 Inflammation in COPD
Dr. Bhatt refers to data from significant studies such as COPD Gene and Eclipse, which show a clear correlation between eosinophil counts and exacerbation frequency in COPD patients. "There's almost a monotonic increase in the frequency of exacerbations with increasing eosinophil count," he notes, highlighting the clinical relevance of these findings. Notably, around 300 cells per microliter marks a significant increase in exacerbation frequency, which continues to rise with higher counts.
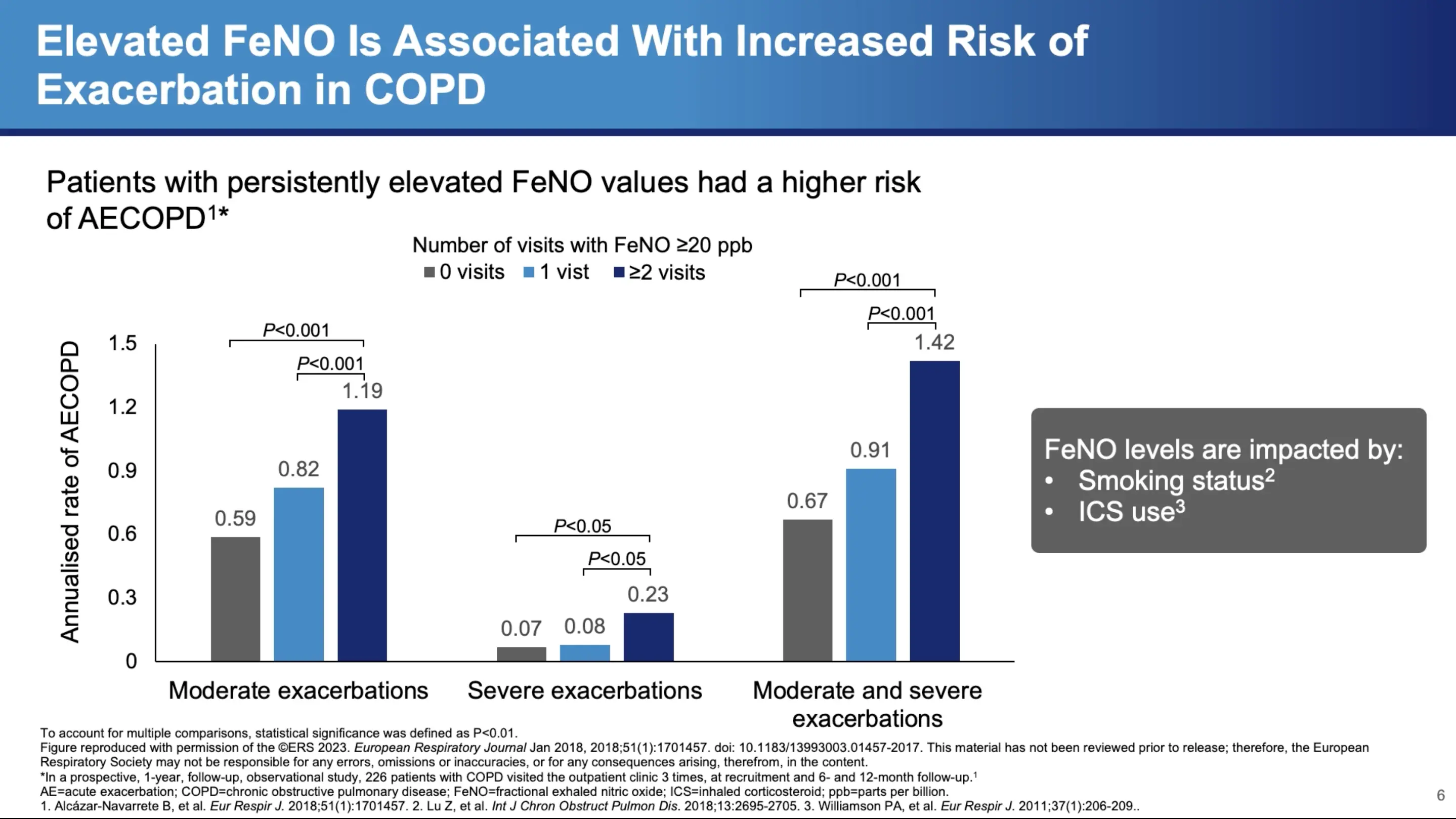
Additionally, other diagnostic tools like fractional exhaled nitric oxide (FeNO) can offer insights into Type 2 inflammation. Dr. Bhatt discusses a study involving 220 patients, which found that varying levels of FeNO were associated with different exacerbation frequencies. Those with persistently elevated FeNO levels showed a continuous increase in exacerbations, suggesting ongoing Type 2 inflammation.
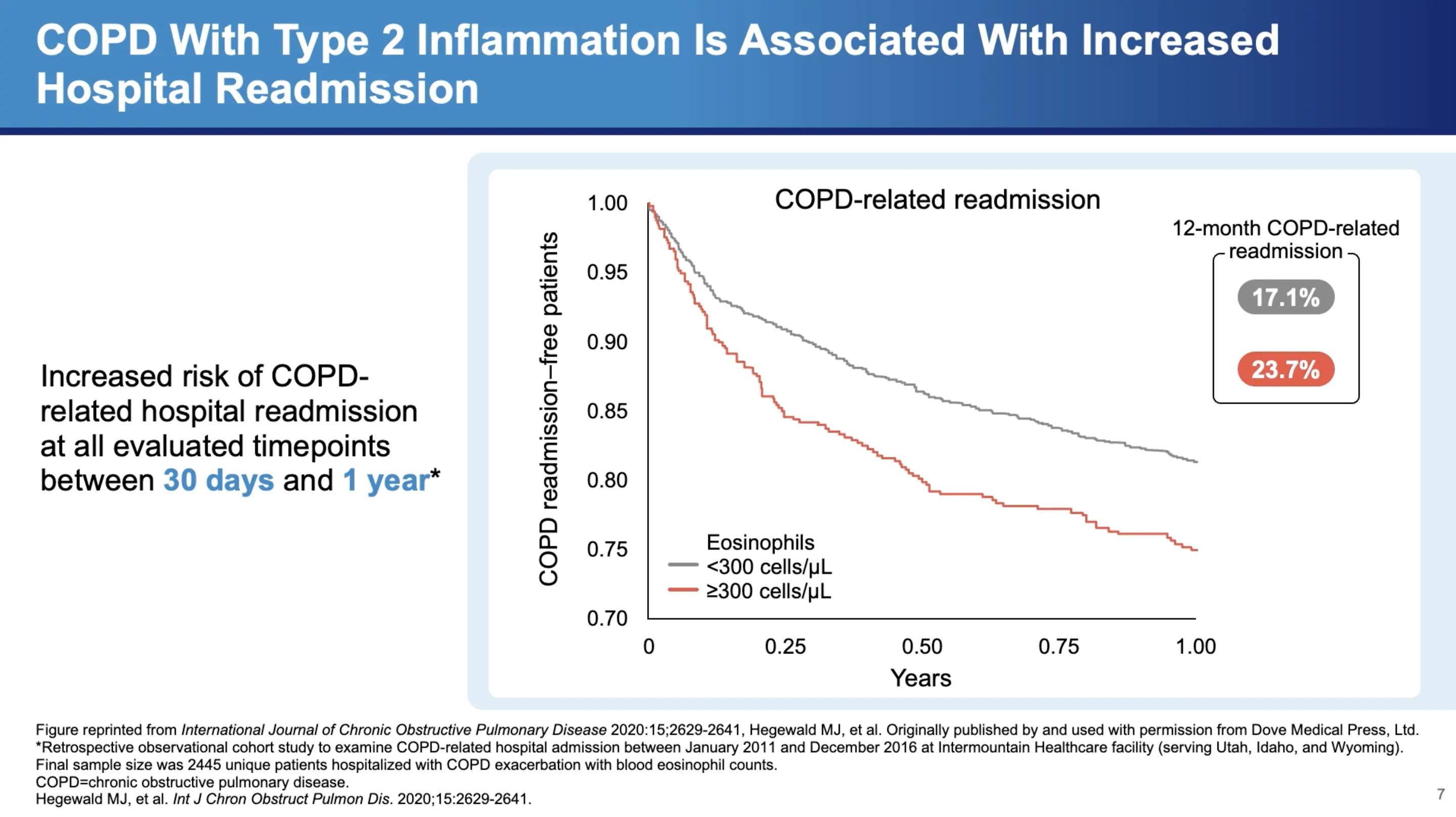
Prognostic Value of Type 2 Inflammation
Type 2 inflammation not only correlates with exacerbation frequency but also has implications for hospital readmissions. Dr. Bhatt cites a retrospective study of almost 2,500 patients from an administrative database, revealing that "high eosinophils, defined as greater than 300 cells per microliter, was associated with a higher COPD-related readmission at 30 days as well as at 52 weeks." This finding underscores the importance of monitoring eosinophil levels for predicting both moderate and severe exacerbations, and potentially tailoring treatment strategies to reduce hospital readmissions.
In Summary
Dr. Surya Bhatt's insights into Type 2 inflammation in COPD highlight a significant shift in understanding and managing this complex disease. Recognizing the role of Type 2 inflammation could lead to more personalized treatment approaches, potentially improving outcomes for a substantial subset of COPD patients. As research continues to evolve, pulmonologists and other healthcare providers must stay informed about these developments to optimize care for their patients with COPD.
In summarizing the impact of these findings, Dr. Bhatt emphasizes the need for continued research and awareness, "I think we need to keep looking," he asserts, pointing towards a future where COPD treatment may become more targeted and effective based on individual inflammatory profiles. This approach could revolutionize the management of COPD, making it not just a matter of managing symptoms but potentially altering the disease course for many patients.
References
- Gandhi NA, Bennett BL, Graham NMH, et al. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016;15(1):35-50.
- Yousuf A, Ibrahim W, Greening NJ, et al. T2 Biologics for Chronic Obstructive Pulmonary Disease. J Allergy Clin Immunol Pract. 2019;7(5):1405-1416.
- Aghapour M, Raee P, Moghaddam SJ, et al. Airway Epithelial Barrier Dysfunction in Chronic Obstructive Pulmonary Disease: Role of Cigarette Smoke Exposure. Am J Respir Cell Mol Biol. 2018;58(2):157-169.
- Barnes JP. Inflammatory endotypes in COPD. Allergy. 2019;74(7):1249-1256.
- Wang Z, Bafadhel M, Haldar K, et al. Lung microbiome dynamics in COPD exacerbations. Eur Respir J. 2016;47(4):1082-1092.
- Smithgall MD, Comeau MR, Yoon BRP, et al. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20(8):1019-1030.
- Senra L, Mylonas A, Kavanagh RD, et al. IL-17E (IL-25) enhances innate immune responses during skin inflammation. J Invest Dermatol. 2019;139(8):1732-1742.
- Linden D, Guo-Parke H, Coyle PV, et al. Respiratory viral infection: a potential “missing link” in the pathogenesis of COPD. Eur Respir Rev. 2019; 28(151):180063.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Accessed July 27, 2023. https://goldcopd.org/2023-gold-report-2/.
- Calderon AA, Dimond C, Choy DF, et al. Targeting interleukin-33 and thymic stromal lymphopoietin pathways for novel pulmonary therapeutics in asthma and COPD. Eur Respir Rev. 2023;32(167):220144.
- Kurokawa M, Matsukura S, Kawaguchi M, et al. Interleukin-33-activated dendritic cells induce the production of thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Arch Allergy Immunol. 2013;161(Suppl 2):52-57.
- Griesenauer B, Paczesny S. The ST2/IL-33 axis in immune cells during inflammatory diseases. Front Immunol. 2017;8:475.
- Borowczyk J, Shutova M, Brembilla NC, et al. IL-25 (IL-17E) in epithelial immunology and pathophysiology. J Allergy Clin Immunol. 2021;148(1):40-52.
- Claudio E, Wang H, Kamenyeva O, et al. IL-25 orchestrates activation of Th cells via conventional dendritic cells in tissue to exacerbate chronic house dust mite–induced asthma pathology. J Immunol. 2019;203(8)2319-2327.
- Kotlyarov S. Involvement of the innate immune system in the pathogenesis of chronic obstructive pulmonary disease. Int J Mol Sci. 2022;23(2):985.
- Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13(5):425-437.
- Saatian B, Rezaee F, Desando S, et al. Interleukin-4 and interleukin-13 cause barrier dysfunction in human airway epithelial cells. Tissue Barriers. 2013;1(2):e24333.
- Zheng T, Zhu Z, Wang Z, et al. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J Clin Invest. 2000;106(9):1081-1093.
- Zhu Z, Homer RJ, Wang Z, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103(6):779-788.
- Alevy YG, Patel AC, Romero AG, et al. IL-13-induced airway mucus production is attenuated by MAPK13 inhibition. J Clin Invest. 2012;122(12):4555-4568.
- Wang X, Xu C, Ji J, et al. IL-4/IL-13 upregulates Sonic hedgehog expression to induce allergic airway epithelial remodeling. Am J Physiol Lung Cell Mol Physiol. 2020;318(5):L888-L899.
- Cooper PR, Poll CT, Barnes PJ, et al. Involvement of IL-13 in tobacco smoke-induced changes in the structure and function of rat intrapulmonary airways. Am J Respir Cell Mol Biol. 2010;43(2):220-226.
- Doyle AD, Mukherjee M, LeSuer WE, et al. Eosinophil-derived IL-13 promotes emphysema. Eur Respir J. 2019;53(5):1801291.
- Sun J, Liu T, Yan Y, et al. The role of Th1/Th2 cytokines played in regulation of specific CD4+ Th1 cell conversion and activation during inflammatory reaction of chronic obstructive pulmonary disease. Scand J Immunol. 2018;88(1):e12674.
- Kim CH, Kim KE, Yoon JH, et al. Upregulation of MUC5AC gene expression by IL-4 through CREB in Human airway epithelial cells. J Cell Biochem. 2009;108(4):974-981.
- Yu H, Li Q, Kolosov VP, et al. Interleukin-13 induces mucin 5AC production involving STAT6/SPDEF in human airway epithelial cells. Cell Commun Adhes. 2010;17(4-6):83-92.
MAT-SA-2400359/V1/Jun/2024