- Article
- Source: Campus Sanofi
- 24 Jun 2024
Blood Eosinophils as a Biomarker in Patients with COPD


Eosinophils in COPD
In patients with COPD, raised eosinophil levels in the blood are associated with higher eosinophil levels in the lungs, which is a biomarker of type 2 inflammatory activity in the airway.1 It has been found that up to 37% of COPD patients show indication of type 2 inflammation.2-5
Raised blood eosinophil levels are detectable by ordering a complete blood count with differential. A large population-based study (N=39,824) evaluated the stability of blood eosinophilia over time. The stability of the blood eosinophil count was observed to be higher in patients with COPD with baseline counts <340 cells/µL vs ≥340 cells/µL. The findings from this study extend the understanding of blood eosinophil as a potential biomarker.5
Raised blood eosinophil count in COPD is associated with poor clinical outcomes and increased healthcare cost
Raised blood eosinophil count in COPD increased the risk of exacerbation,6 1-year COPD-related readmission,7 and severe exacerbations8 as shown in the illustration below:
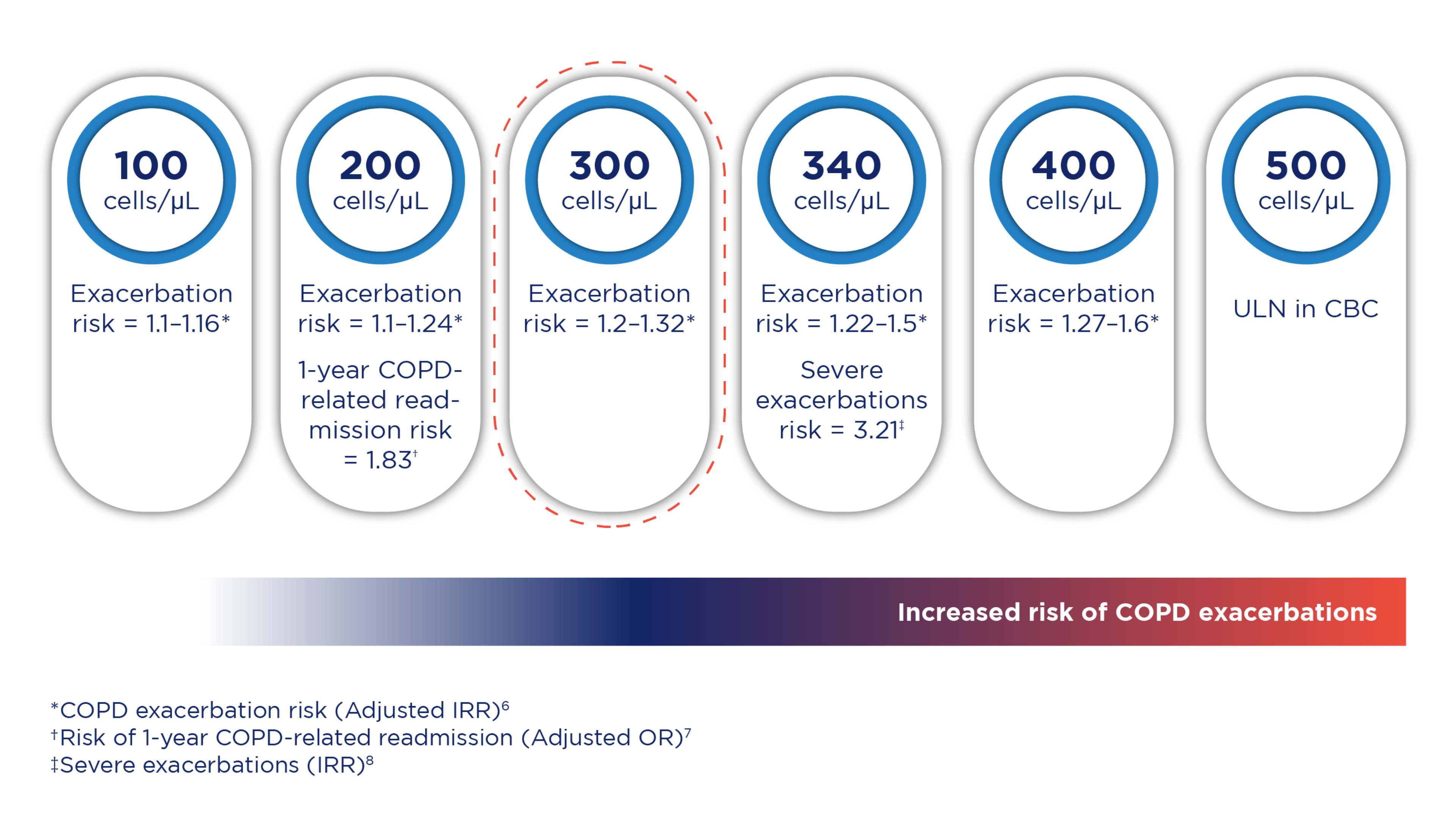
COPD exacerbation risk increases with higher eosinophil counts. In the COPDGene study, COPD patients with blood eosinophil count ≥300 cells/μL demonstrated an increased risk of exacerbations. This was prospectively validated in the ECLIPSE study.6
Type 2 inflammation in COPD (Eosinophilic COPD) and its risks
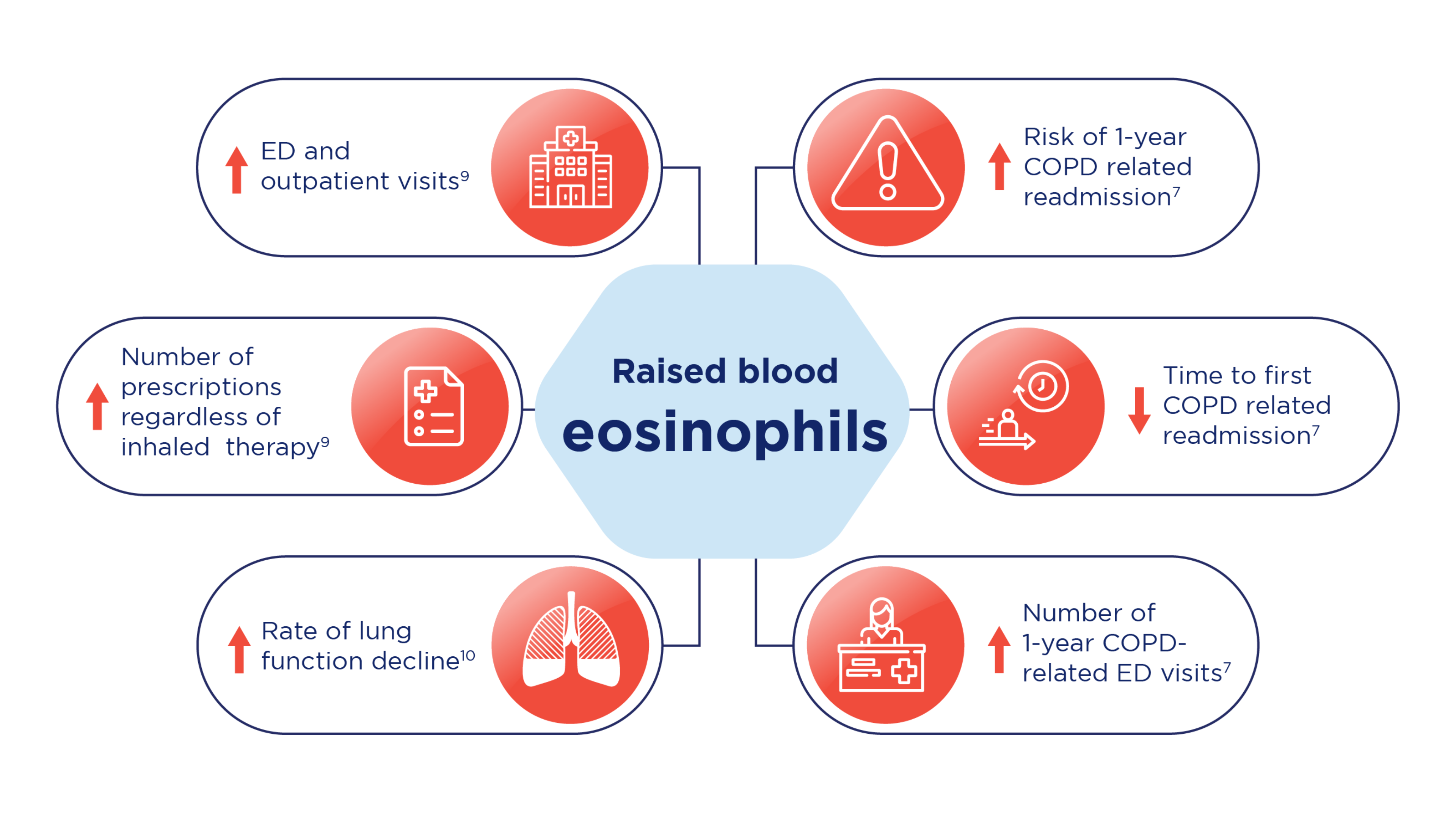
Raised blood eosinophils are associated with higher emergency department visits, outpatient visits, numbers of prescriptions* and increased rate of lung function decline.9,10 Raised eosinophils in COPD may also lead to increased risk of 1-year COPD-related readmission, decreased time to first COPD-related readmission and increased number of 1-year COPD-related Emergency Department (ED) visits7 as shown in the illustration and table below:
Risks associated with raised blood eosinophil levels
To learn more about the impact of raised eosinophiles in COPD read this article
An Overview of the impact of Type 2 Inflammation in COPD: Insights from Dr. Surya Bhatt
Impact of blood eosinophil count in COPD on management decisions

Initial pharmacological management (Group E†)1:
GOLD references suggest that ICS should be added to long-acting bronchodilators therapy in patients with high blood eosinophils counts (≥300 cells/µL). Consider triple therapy (LABA+LAMA+ICS) if eosinophil count is ≥300 cells/μL and this is based on the effect of ICS on exacerbation prevention correlated to blood eosinophil count. There is no direct data on initiation with triple therapy in newly diagnosed patients. However, available studies in treated patients provide a rationale to initiate treatment with triple therapy in patients with high eosinophils counts (≥300 cells/µL).1
Blood eosinophil count impact on initial management decisions, according to GOLD references1

Follow-up pharmacological management:
Exacerbations:1
GOLD references recommend escalation to LABA + LAMA for patients with persistent exacerbations on bronchodilator therapy.
ICS is suggested to be added to LABA + LAMA in patients who develop further exacerbations. Beneficial response of ICS may be observed at blood eosinophil counts of ≥100 cells/μL.
For patients treated with LABA + LAMA + ICS or those with eosinophil counts of <100 cells/μL who still have exacerbations — Please refer to GOLD guidelines for more information
Blood eosinophil count impact on follow-up pharmalogical management decisions, according to GOLD1


*Regardless of inhaled therapy
†Group “E” are patients with frequent exacerbations (defined as ≥2 in the past 12 months or 1 exacerbation requiring hospitalization).
COPD, chronic obstructive pulmonary disease; ED, emergency department; FEV1, forced expiratory volume in first second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HR, hazard ratio; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; OR, odds ratio; IRR, incidence rate ratio; ULN, upper limit of normal.
References
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2024 report). Accessed [April 21, 2024]. https://goldcopd.org/wp-content/uploads/2024/02/GOLD-2024_v1.2-11Jan24_WMV.pdf.
- Casanova C, Celli BR, de-Torres JP, et al. Prevalence of persistent blood eosinophilia: relation to outcomes in patients with COPD. Eur Respir J. 2017;50:1701162. doi:10.1183/13993003.01162-2017
- Singh D, Kolsum U, Brightling CE, Locantore N, Agusti A, Tal-Singer R; ECLIPSE investigators. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44(6):1697-1700.
- Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184(6):662-671.
- Oshagbemi OA, Burden AM, Braeken DCW, et al. Stability of blood eosinophils in patients with chronic obstructive pulmonary disease and in control subjects, and the impact of sex, age, smoking, and baseline counts. Am J Respir Crit Care Med. 2017;195(10):1402-1404.
- Yun JH, Lamb A, Chase R, et al. Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141(6):2037–2047.
- Bélanger M, Couillard S, Courteau J et al. Eosinophil counts in first COPD hospitalizations: a comparison of health service utilization. Int J Chron Obstruct Pulmon Dis. 2018;13:3045–3054.
- Vedel-Krogh S, Nielsen SF, Lange P et al. Blood Eosinophils and Exacerbations in Chronic Obstructive Pulmonary Disease. The Copenhagen General Population Study. Am J Respir Crit Care Med. 2016; 193:965–974.
- Müllerova H, Hahn B, Simard EP et al. Exacerbations and health care resource use among patients with COPD in relation to blood eosinophil counts. Int J Chron Obstruct Pulmon Dis. 2019;14:683–696.
- Tan WC, Bourbeau J, Nadeau G et al. High eosinophil counts predict decline in FEV1: Results from the CanCOLD study. Eur Respir J 2021; 57(5):2000838
MAT-SA-2400418/V1/SEP24