- Article
- Source: Campus Sanofi
- 16 May 2024
The burden and Risk of Exacerbations in COPD according to Dr. Surya Bhatt

What is COPD?
COPD is a chronic inflammatory lung disease that causes obstructed airflow from the lungs. Symptoms include breathing difficulty, cough, mucus (sputum) production, and wheezing. It is typically caused by long-term exposure to irritating gases or particulate matter, most often from cigarette smoke. Individuals with COPD are at increased risk of developing heart disease, lung cancer, and a variety of other conditions.
The Critical Role of Exacerbations in COPD Progression
Dr. Bhatt emphasizes that acute exacerbations of COPD are not merely inconveniences but are pivotal events that significantly influence the course of the disease. "One of the other things that's very important in COPD is acute exacerbations. And these are not just an inconvenience but are associated with significant disease progression," he states. Exacerbations are typically triggered by infections such as viruses and bacterial infections or by environmental pollution and occupational exposures.
Impact on Lung Function
Exacerbations have a profound impact on lung function, with data suggesting a link between exacerbations and accelerated lung function decline. Dr. Bhatt notes, "There's plenty of data to suggest that these exacerbations are associated with lung function decline." This decline is more severe in the case of frequent exacerbations. For instance, he cites the COPD Gene study, which shows how the frequency of exacerbations correlates with the rate of FEV1 decline—a measure of lung function.
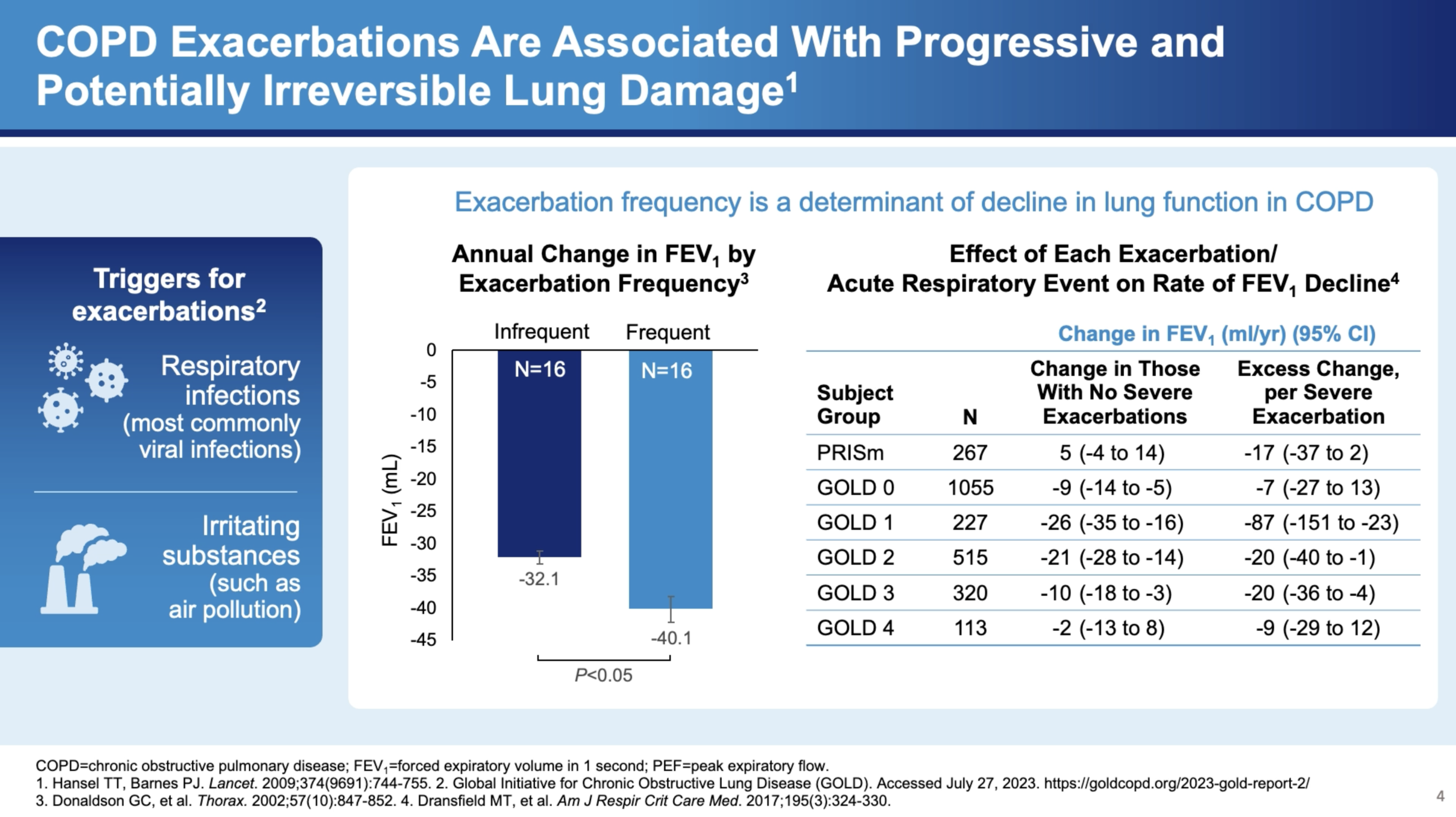
Severity and Treatment
The severity of exacerbations can vary, significantly affecting treatment approaches and outcomes. "But if they manage to adjust steroids and antibiotics at home, that classifies as a moderate exacerbation. But if it's severe enough to end up in the hospital, then that causes a severe exacerbation," explains Dr. Bhatt. The distinction between moderate and severe exacerbations is crucial as it determines the immediate treatment protocol and the long-term management strategy.
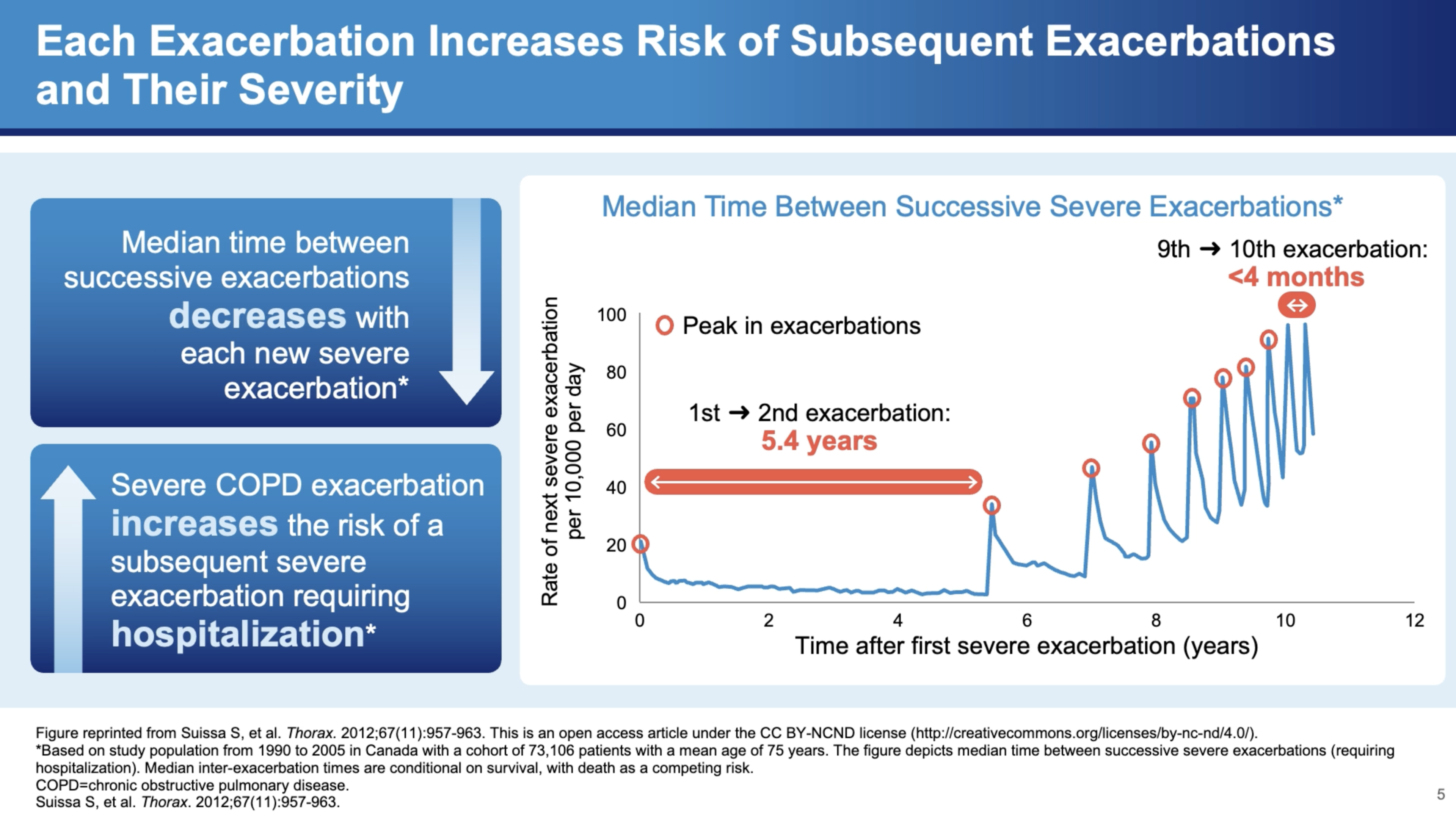
The Cascading Effects of Severe Exacerbations
Severe exacerbations are particularly concerning due to their cascading effects on health. Dr. Bhatt discusses a study by Sammy Sosa, which modeled severe exacerbation frequency among approximately 75,000 patients in Canada. The study found that the interval between exacerbations tends to decrease over time, indicating a worsening pattern of the disease. "When somebody gets a first exacerbation, it seems like it begets more exacerbations," he explains.
Mortality Risks
The mortality risks associated with severe exacerbations are stark. Dr. Bhatt highlights, "The hazards of mortality is about twice if you get two severe exacerbations as opposed to one severe exacerbation. And the hazards of mortality is fivefold if you get ten exacerbations as opposed to one severe exacerbation." Furthermore, the risk of dying within one year of a severe exacerbation requiring hospitalization is about 25%, which increases to 50% within four years after discharge.
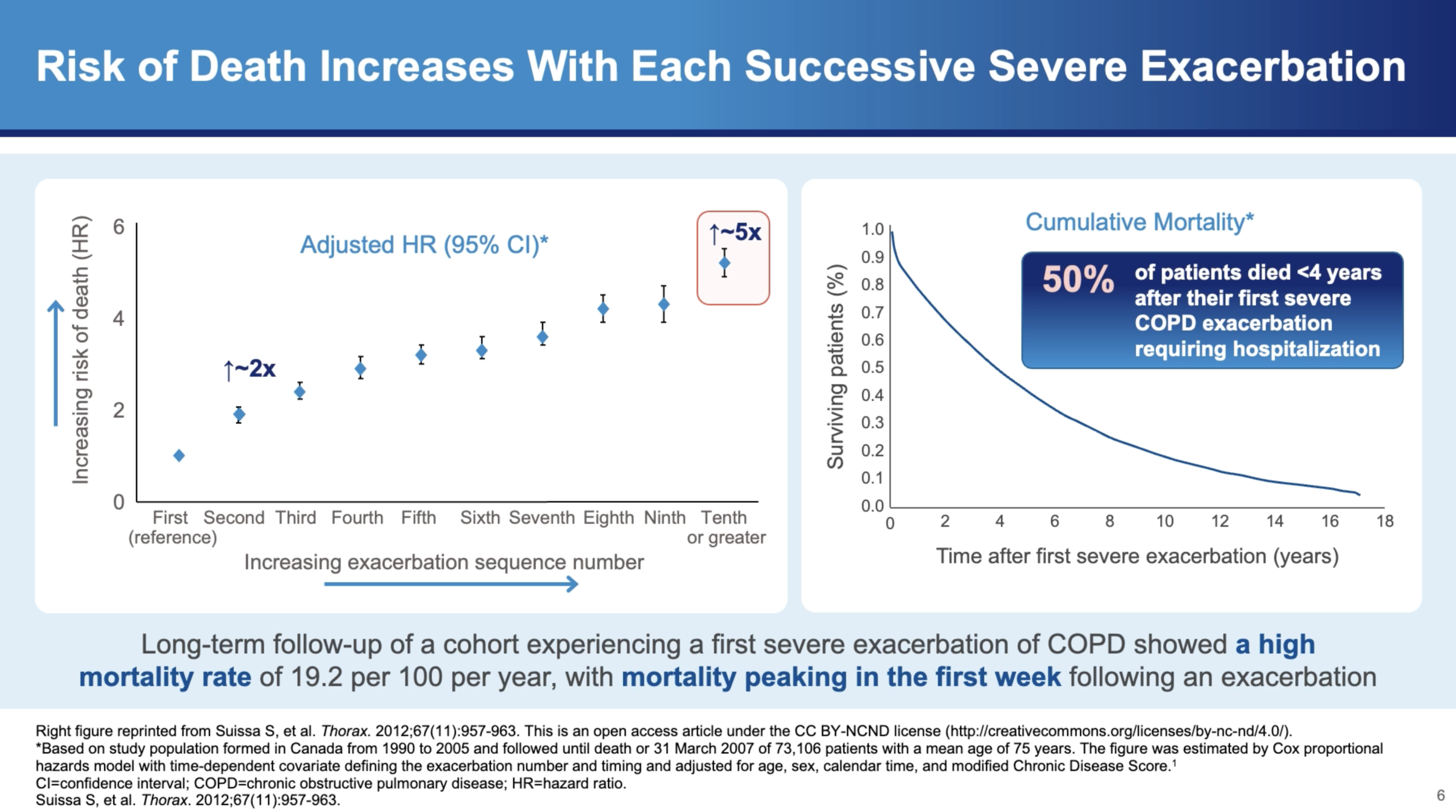
In Summary
The insights provided by Dr. Surya Bhatt illuminate the severe implications of COPD exacerbations. Understanding the triggers, frequency, and severity of these exacerbations is crucial for managing COPD effectively. As research continues to evolve, it is essential for healthcare providers to stay informed about the best practices for treating and managing exacerbations to improve the quality of life for COPD patients. Additionally, preventative strategies, including smoking cessation and reducing exposure to pollutants, remain key components of COPD management. Through a comprehensive approach that includes both prevention and effective exacerbation management, the impact of COPD can be significantly mitigated.
References
- Gandhi NA, Bennett BL, Graham NMH, et al. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016;15(1):35-50.
- Yousuf A, Ibrahim W, Greening NJ, et al. T2 Biologics for Chronic Obstructive Pulmonary Disease. J Allergy Clin Immunol Pract. 2019;7(5):1405-1416.
- Aghapour M, Raee P, Moghaddam SJ, et al. Airway Epithelial Barrier Dysfunction in Chronic Obstructive Pulmonary Disease: Role of Cigarette Smoke Exposure. Am J Respir Cell Mol Biol. 2018;58(2):157-169.
- Barnes JP. Inflammatory endotypes in COPD. Allergy. 2019;74(7):1249-1256.
- Wang Z, Bafadhel M, Haldar K, et al. Lung microbiome dynamics in COPD exacerbations. Eur Respir J. 2016;47(4):1082-1092.
- Smithgall MD, Comeau MR, Yoon BRP, et al. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20(8):1019-1030.
- Senra L, Mylonas A, Kavanagh RD, et al. IL-17E (IL-25) enhances innate immune responses during skin inflammation. J Invest Dermatol. 2019;139(8):1732-1742.
- Linden D, Guo-Parke H, Coyle PV, et al. Respiratory viral infection: a potential “missing link” in the pathogenesis of COPD. Eur Respir Rev. 2019; 28(151):180063.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Accessed July 27, 2023. https://goldcopd.org/2023-gold-report-2/.
- Calderon AA, Dimond C, Choy DF, et al. Targeting interleukin-33 and thymic stromal lymphopoietin pathways for novel pulmonary therapeutics in asthma and COPD. Eur Respir Rev. 2023;32(167):220144.
- Kurokawa M, Matsukura S, Kawaguchi M, et al. Interleukin-33-activated dendritic cells induce the production of thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Arch Allergy Immunol. 2013;161(Suppl 2):52-57.
- Griesenauer B, Paczesny S. The ST2/IL-33 axis in immune cells during inflammatory diseases. Front Immunol. 2017;8:475.
- Borowczyk J, Shutova M, Brembilla NC, et al. IL-25 (IL-17E) in epithelial immunology and pathophysiology. J Allergy Clin Immunol. 2021;148(1):40-52.
- Claudio E, Wang H, Kamenyeva O, et al. IL-25 orchestrates activation of Th cells via conventional dendritic cells in tissue to exacerbate chronic house dust mite–induced asthma pathology. J Immunol. 2019;203(8)2319-2327.
- Kotlyarov S. Involvement of the innate immune system in the pathogenesis of chronic obstructive pulmonary disease. Int J Mol Sci. 2022;23(2):985.
- Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13(5):425-437.
- Saatian B, Rezaee F, Desando S, et al. Interleukin-4 and interleukin-13 cause barrier dysfunction in human airway epithelial cells. Tissue Barriers. 2013;1(2):e24333.
- Zheng T, Zhu Z, Wang Z, et al. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J Clin Invest. 2000;106(9):1081-1093.
- Zhu Z, Homer RJ, Wang Z, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103(6):779-788.
- Alevy YG, Patel AC, Romero AG, et al. IL-13-induced airway mucus production is attenuated by MAPK13 inhibition. J Clin Invest. 2012;122(12):4555-4568.
- Wang X, Xu C, Ji J, et al. IL-4/IL-13 upregulates Sonic hedgehog expression to induce allergic airway epithelial remodeling. Am J Physiol Lung Cell Mol Physiol. 2020;318(5):L888-L899.
- Cooper PR, Poll CT, Barnes PJ, et al. Involvement of IL-13 in tobacco smoke-induced changes in the structure and function of rat intrapulmonary airways. Am J Respir Cell Mol Biol. 2010;43(2):220-226.
- Doyle AD, Mukherjee M, LeSuer WE, et al. Eosinophil-derived IL-13 promotes emphysema. Eur Respir J. 2019;53(5):1801291.
- Sun J, Liu T, Yan Y, et al. The role of Th1/Th2 cytokines played in regulation of specific CD4+ Th1 cell conversion and activation during inflammatory reaction of chronic obstructive pulmonary disease. Scand J Immunol. 2018;88(1):e12674.
- Kim CH, Kim KE, Yoon JH, et al. Upregulation of MUC5AC gene expression by IL-4 through CREB in Human airway epithelial cells. J Cell Biochem. 2009;108(4):974-981.
- Yu H, Li Q, Kolosov VP, et al. Interleukin-13 induces mucin 5AC production involving STAT6/SPDEF in human airway epithelial cells. Cell Commun Adhes. 2010;17(4-6):83-92.
MAT-SA-2400359/V1/Jun/2024