- Article
- Source: Campus Sanofi
- Sep 26, 2024
A review of the clinical implications of eosinophil stability and predictive capability in COPD
Do blood eosinophils have a role in COPD?
In COPD, raised blood eosinophils (EOS) levels are associated with type 2 inflammation1 and have been linked to a higher risk of exacerbations.2 Understanding the role of EOS levels and their evolution through time can be important for clinicians to phenotype their patients and evaluate potential risk of exacerbation.3 However, a look-back period may be warranted while assessing EOS levels in patients because EOS levels may fluctuate as a result of treatment.4
Discover more about the Role of type 2 Inflammation in COPD
Eosinophilic inflammation endotype
Eosinophilic inflammation is a stable endotype. The AERIS study investigated the stability of this phenotype over time. The study results demonstrated that eosinophilic inflammation (blood EOS count ≥2%) is common at the time of exacerbation in patients with predominantly raised EOS during stable state.5 The likelihood of an exacerbation being eosinophilic was nine folds higher in patients with blood EOS ≥2% than in those with blood EOS <2% at enrolment (OR = 9.16; 95% CI: 4.10–20.47; P <0.001).5
What is known about EOS level stability?
Temporal stability of EOS Levels
The concept of temporal stability refers to the consistency of a biomarker over time.6 In a study by Landis et al., the blood EOS counts taken during stable COPD were reasonably reproducible.7
A study by Long et al. provides robust data on the temporal stability of EOS levels in COPD patients. In their analysis of 225 patients from the COPDMAP cohort, Long and colleagues investigated EOS stability over 1 year using 2019 GOLD COPD thresholds (<100, 100–<300 and ≥300 EOS/μL).8 They found that 69.3% of patients had good stability of EOS levels from baseline to 1 year (measurements taken during stable state) within the same 2019 GOLD COPD thresholds.8 This temporal stability indicates that EOS levels measured during the stable phase of COPD are not random fluctuations but reflect an underlying and consistent inflammatory phenotype. Moreover, the study demonstrated that patients (no exacerbation, 66.7% patients; ≥1 exacerbation, 72.7% patients; ≥2 exacerbations, 74.3% patients) had stable blood EOS levels over 1 year when stratified into subgroups by number of exacerbations within a year.8
Association between EOS levels and future exacerbation risk9,10
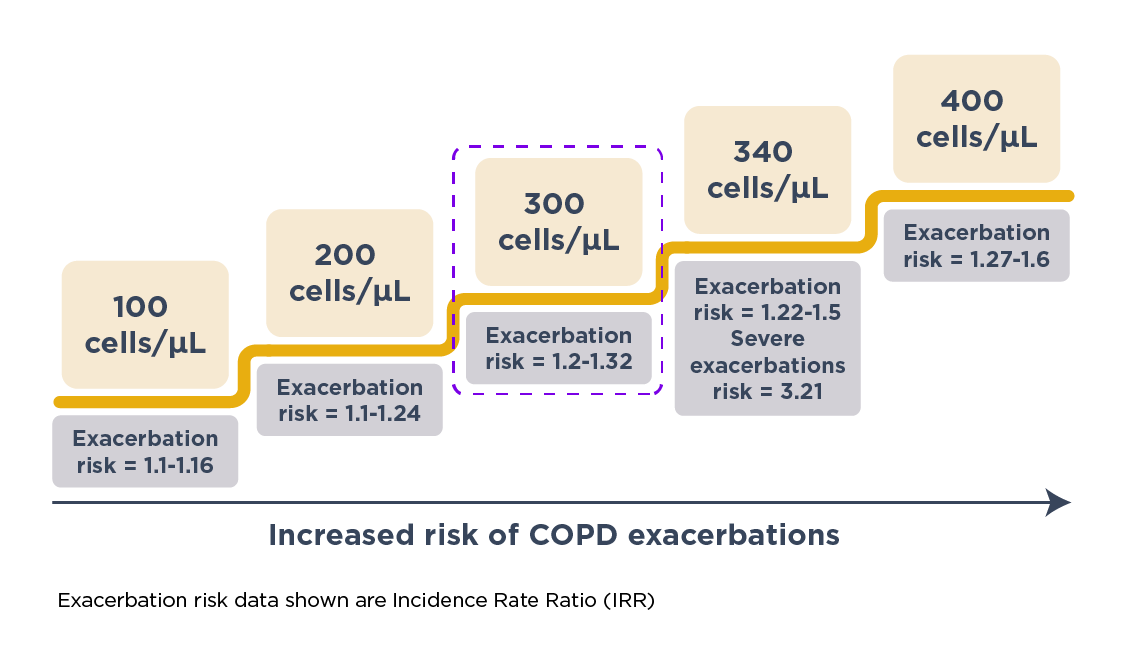
Yun et al. prospectively validated the association of increased EOS levels with exacerbations in COPD in the ECLIPSE study.9 In this study, the researchers measured EOS levels during the stable phase and tracked the occurrence of exacerbations over a 3-year period.9 They found that raised EOS level (≥300 cells/μL) at baseline during the stable phase was a consistent predictor of risk of exacerbations in the following year (IRR = 1.20; 95% CI: 1.05–1.36 at 1 year).9 Specifically, the exacerbation risk was higher in patients with persistently raised (vs low) EOS levels, indicating that such patients had the greatest risk. Furthermore, the study revealed that the predictive capability of EOS levels extended beyond just a year (IRR = 1.22; 95% CI: 1.06–1.42 for overall study period).9 Patients with high EOS levels were consistently more likely to exacerbate over 3 years. This extended predictive capability underscores the utility of EOS level as a long-term predictor of exacerbation risk, allowing for early and proactive management strategies.9

Impact of oral corticosteroids use on blood EOS levels in patients with COPD
An inverse linear relationship between blood EOS levels and oral corticosteroids (OCS) dose was observed by Prazma et al. in patients with severe asthma, with an increase in blood EOS levels by 41% for every 5 mg/day decrease in OCS dose.11 To our knowledge, the impact of OCS use on blood EOS levels has not been studied in patients with COPD. However, OCS use is known to suppress EOS levels in patients with COPD.12 A study reported significant reduction in sputum EOS counts of six folds, following daily OCS use for 2 weeks.13 Measurement of EOS levels in patients whose levels are suppressed due to OCS administration could give an inaccurate assessment of EOS levels.12
Learn more about Blood Eosinophils as a Biomarker in Patients with COPD
What are the clinical implications of EOS stability and predictive capability?
The evidence supporting the EOS stability8 and long-term predictive capability9 of EOS levels in COPD has significant clinical implications. By identifying patients with consistently raised EOS levels, healthcare providers can stratify patients based on their risk of future exacerbations. This stratification can guide treatment decisions, particularly the use of OCS,14 inhaled (ICS)15 or biologics16 that target type 2 inflammation. For instance, a COPD patient with consistently high EOS levels (>300 cells/μL) during the stable phase may be an ideal candidate for early intervention with ICS,15 which has been shown to reduce the frequency and severity of exacerbations in eosinophilic COPD.17,18 On the contrary, absence of raised blood EOS levels of >2% may help identify a subgroup with an adverse impact of OCS on their recovery.14 The stability of EOS levels over time allows clinicians to monitor these levels as part of routine care, making it easier to identify changes in the patient’s inflammatory profile and adjust treatment accordingly.
For further guidance on how to manage COPD patients please refer to the GOLD Recommendations for Treatment and Management of COPD

The long-term predictive capability of blood EOS levels9 is particularly useful, as it enables healthcare providers to anticipate and mitigate the risk of exacerbations, which are a major driver of morbidity and healthcare costs in COPD.14,19,20
How can EOS measurements be incorporated into clinical practice?
Incorporating EOS measurements into routine COPD management requires careful consideration of the data thresholds and the specific clinical context. The threshold of ≥300 cells/μL is commonly used to define raised EOS levels,9 based on evidence from multiple studies, including that by Yun et al.9 However, some variability exists in the optimal threshold depending on the patient population and the specific outcomes being predicted.21
In practice, measuring EOS levels during the stable phase of COPD can be easily integrated into routine blood tests.22 Given the evidence of temporal stability, a single measurement can provide a reliable assessment of the patient’s eosinophilic phenotype.7 Patients with raised EOS levels should be closely monitored for signs of exacerbation,23 and consideration should be given to the early initiation of ICS.1 Currently various clinical trials are in progress to evaluate the role of therapies targeting type 2 inflammation in COPD with raised EOS levels.24
Moreover, the predictive capability of EOS levels means that these measurements can inform long-term management strategies. For instance, patients with high baseline EOS levels may require more intensive follow-up and a lower threshold for escalating treatment in the event of early signs of exacerbation. This proactive approach can help reduce the frequency and severity of exacerbations, ultimately improving patient outcomes and reducing the burden on healthcare systems.22
Challenges and future directions
While the evidence supporting the use of EOS levels as a biomarker in COPD is compelling, several challenges remain. One challenge is the variability in EOS levels due to external factors such as infections,25 medications,21 and environmental exposures.26 These factors can influence EOS levels and potentially confound their predictive value. Therefore, it is essential for clinicians to interpret EOS measurements in the context of the patient’s overall clinical picture and consider repeat measurements if necessary.
Another challenge is the need for further research to refine the thresholds for EOS levels in different clinical scenarios. While the ≥300 cells/μL threshold is widely used, there is a need for more data to establish the optimal cut-off points for different patient populations and to validate these thresholds in diverse clinical settings.23
Future research should also explore the potential for combining EOS levels with other biomarkers and clinical features to enhance predictive accuracy. For example, integrating EOS measurements with markers of airway inflammation27 or with clinical features such as exacerbation history,28 could provide a more comprehensive risk assessment tool for COPD patients.
References
- Singh D. Blood eosinophil counts in chronic obstructive pulmonary disease: a biomarker of inhaled corticosteroid effects. Tuber Respir Dis (Seoul). 2020;83(3):185–194. Doi: 10.4046/trd.2020.0026. PMID: 32578413.
- Komura M, Sato T, Suzuki Y, Yoshikawa H, Nitta NA, Hayashi M, Kawasaki E, Horikoshi K, Nishioki T, Mori M, Kodama Y, Sasaki S, Takahashi K. Blood Eosinophil Count as a Predictive Biomarker of Chronic Obstructive Pulmonary Disease Exacerbation in a Real-World Setting. Can Respir J. 2023 May 25;2023:3302405. doi: 10.1155/2023/3302405. PMID: 37275320; PMCID: PMC10234729.
- David B, Bafadhel M, Koenderman L, De Soyza A. Eosinophilic inflammation in COPD: from an inflammatory marker to a treatable trait. Thorax. 2021 Feb;76(2):188-195. doi: 10.1136/thoraxjnl-2020-215167. Epub 2020 Oct 29. PMID: 33122447; PMCID: PMC7815887.
- Sivapalan P, Bikov A, Jensen JU. Using Blood Eosinophil Count as a Biomarker to Guide Corticosteroid Treatment for Chronic Obstructive Pulmonary Disease. Diagnostics (Basel). 2021 Feb 3;11(2):236. doi: 10.3390/diagnostics11020236. PMID: 33546498; PMCID: PMC7913607.
- Kim VL, Coombs NA, Staples KJ, Ostridge KK, Williams NP, Wootton SA, Devaster JM, Aris E, Clarke SC, Tuck AC, Bourne SC. Impact and associations of eosinophilic inflammation in COPD: analysis of the AERIS cohort. Eur Respir. 2017;50(4). doi: 10.1183/13993003.00853-2017. PMID: 29025891.
- Walsh CP, Lindsay EK, Grosse P, Natale BN, Fairlie S, Bwint A, Schaffer L, McMahon K, Del Duke C, Forse J, Lamonja-Vicente N. A systematic review and meta-analysis of the stability of peripheral immune markers in healthy adults. Brain Behav Immun.2023;107:32–46. doi: 10.1016/j.bbi.2022.09.011. PMID: 36152782.
- Landis SH, Suruki R, Hilton E, Compton C, Galwey NW. Stability of blood eosinophil count in patients with COPD in the UK clinical practice research datalink. COPD. 2017;14(4):382–388. doi: 10.1080/15412555.2017.1313827. PMID: 28569614.
- Long GH, Southworth T, Kolsum U, Donaldson GC, Wedzicha JA, Brightling CE, Singh D. The stability of blood Eosinophils in chronic obstructive pulmonary disease. Respir Res.2020;21:1–4. doi: 10.1186/s12931-020-1279-4. PMID: 31924207.
- Yun JH, Lamb A, Chase R, Singh D, Parker MM, Saferali A, Vestbo J, Tal-Singer R, Castaldi PJ, Silverman EK, Hersh CP. Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol.2018;141(6):2037–2047. doi: 10.1016/j.jaci.2018.04.010. PMID: 29709670.
- Vedel-Krogh S, Nielsen SF, Lange P, Vestbo J, Nordestgaard BG. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease. The Copenhagen General Population Study. Am J Respir Crit Care Med.2016 May 1;193(9):965–74. doi: 10.1164/rccm.201509-1869OC. PMID: 26641631.
- Prazma CM, Bel EH, Price RG, Bradford ES, Albers FC, Yancey SW. Oral corticosteroid dose changes and impact on peripheral blood eosinophil counts in patients with severe eosinophilic asthma: a post hoc analysis. Respir Res. 2019 May 3;20(1):83. doi: 10.1186/s12931-019-1056-4. PMID: 31053134; PMCID: PMC6499981.
- Mathioudakis AG, Bikov A, Foden P, Lahousse L, Brusselle G, Singh D, Vestbo J. Change in blood eosinophils following treatment with inhaled corticosteroids may predict long-term clinical response in COPD. Eur Respir J. 2020 May 27;55(5):1902119. doi: 10.1183/13993003.02119-2019. PMID: 32108044.
- Brightling CE, Monteiro W, Ward R, Parker D, Morgan MD, Wardlaw AJ, Pavord ID. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2000 Oct 28;356(9240):1480-5. doi: 10.1016/S0140-6736(00)02872-5. PMID: 11081531.
- Bafadhel M, McKenna S, Terry S, Mistry V, Pancholi M, Venge P, Lomas DA, Barer MR, Johnston SL, Pavord ID, Brightling CE. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med.2012;186(1):48–55. doi: 0.1164/rccm.201108-1553OC. PMID: 22447964.
- Lea S, Higham A, Beech A, Singh D. How inhaled corticosteroids target inflammation in COPD. Eur Respir Rev.2023;32(170). doi: 10.1183/16000617.0084–2023. PMID: 37852657.
- Ohnishi H, Eitoku M, Yokoyama A. A systematic review and integrated analysis of biologics that target Type 2 inflammation to treat COPD with increased peripheral blood eosinophils. Heliyon. 2022;8(6). doi: 10.1016/j.heliyon.2022.e09736. PMID: 35756113.
- Quint JK, Ariel A, Barnes PJ. Rational use of inhaled corticosteroids for the treatment of COPD. NPJ Prim Care Respir Med. 2023;33(1):27. doi: 10.1038/s41533-023-00347-6. PMID: 37488104.
- Mkorombindo T, Dransfield MT. Inhaled corticosteroids in chronic obstructive pulmonary disease: benefits and risks. Clin Chest Med. 2020;41(3):475–484. doi: 10.1016/j.ccm.2020.05.006. PMID: 32800200.
- Ritchie AI, Wedzicha JA. Definition, causes, pathogenesis, and consequences of chronic obstructive pulmonary disease exacerbations. Clin Chest Med.2020;41(3):421–438. doi: 10.1016/j.ccm.2020.06.007. PMID: 32800196.
- Bafadhel M, Davies L, Calverley PM, Aaron SD, Brightling CE, Pavord ID. Blood eosinophil guided prednisolone therapy for exacerbations of COPD: a further analysis. Eur Respir. 2014;44(3):789–91. doi: 10.1183/09031936.00062614. PMID: 24925917.
- Benson VS, Hartl S, Barnes N, Galwey N, Van Dyke MK, Kwon N. Blood eosinophil counts in the general population and airways disease: a comprehensive review and meta-analysis. Eur Respir J. 2022;59(1). doi: 10.1183/09031936.00062614. PMID: 24925917.
- Bartziokas K, Gogali A, Kostikas K. The role of blood eosinophils in the management of COPD: an attempt to answer the important clinical questions. COPD. 2021;18(6):690–699. doi: 10.1080/15412555.2021.1985989. PMID: 34657541.
- Oliver B, Tonga K, Darley D, Rutting S, Zhang X, Chen H, Wang G. COPD treatment choices based on blood eosinophils: are we there yet?. Breathe (Sheff). 2019;15(4):318–323. doi: 10.1183/20734735.0254-2019. PMID: 31803266.
- Rabe KF, Rennard S, Martinez FJ, Celli BR, Singh D, Papi A, Bafadhel M, Heble J, Radwan A, Soler X, Jacob Nara JA. Targeting type 2 inflammation and epithelial alarmins in chronic obstructive pulmonary disease: a biologics outlook. Am J Respir Crit Care Me. 2023 Aug 15;208(4):395–405. doi: 10.1164/rccm.202303-0455CI. PMID: 37348121.
- Roufosse F, Weller PF. Practical approach to the patient with hypereosinophilia. Allergy Clin Immunol.2010 Jul 1;126(1):39–44. doi: 10.1016/j.jaci.2010.04.011. PMID: 20538328.
- Chipps BE, Jarjour N, Calhoun WJ, Iqbal A, Haselkorn T, Yang M, Brumm J, Corren J, Holweg CT, Bafadhel M. A comprehensive analysis of the stability of blood eosinophil levels. Ann Am Thorac Soc. 2021;18(12):1978–1987. doi: 10.1513/AnnalsATS.202010-1249OC. PMID: 33891831.
- Oishi K, Matsunaga K, Shirai T, Hirai K, Gon Y. Role of Type2 Inflammatory Biomarkers in Chronic Obstructive Pulmonary Disease. J Clin Med. 2020 Aug 18;9(8):2670. doi: 10.3390/jcm9082670. PMID: 32824775; PMCID: PMC7464674.
- Haughney J, Lee AJ, Nath M, Müllerová H, Holmgren U, Nigris ED, Ding B. The long-term clinical impact of COPD exacerbations: a 3-year observational study (SHERLOCK). Ther Adv Respir Dis.2022;16:17534666211070139. doi: 10.1177/17534666211070139. PMID: 35156488.
MAT-GLB-2400917