- Article
- Source: Campus Sanofi
- Aug 28, 2024
What tests can help diagnose and monitor your patients with COPD?


COPD diagnosis is suspected in a patient with symptoms of dyspnea, chronic cough, or sputum production. Key clinical indicators that increase the probability of a COPD diagnosis are progressive persistent dyspnea, worsening with exercise, recurrent wheezing, chronic cough, recurrent lower respiratory infections and a history of risk factors. However, spirometry is required to establish COPD diagnosis.1
COPD needs to be accurately diagnosed for optimal management. Therefore, differential diagnoses, such as asthma, congestive heart failure, bronchiectasis, tuberculosis, obliterative bronchiolitis, and diffuse panbronchiolitis, should be considered to make a clear distinction from COPD.1 Asthma is difficult to distinguish from COPD by current imaging modalities or physiological tests in some patients.1
View this article to know how to differentiate between COPD and asthma.
After establishment of COPD diagnosis, further assessment is required to guide treatment. The goals of initial assessment of COPD are to determine the severity of airflow obstruction, impact of disease on patient’s health status, and risk of future events. 1
There are various tools for COPD monitoring. Read this article to find out more about the key tools to measure the success of COPD management.
Spirometry
Spirometry is one of the useful tools for pulmonary function test for COPD.2 It is readily available, non-invasive, reproducible and objective.1 During early stages of COPD when respiratory symptoms may be absent, spirometry can help identify COPD.1
Key variables associated with spirometry
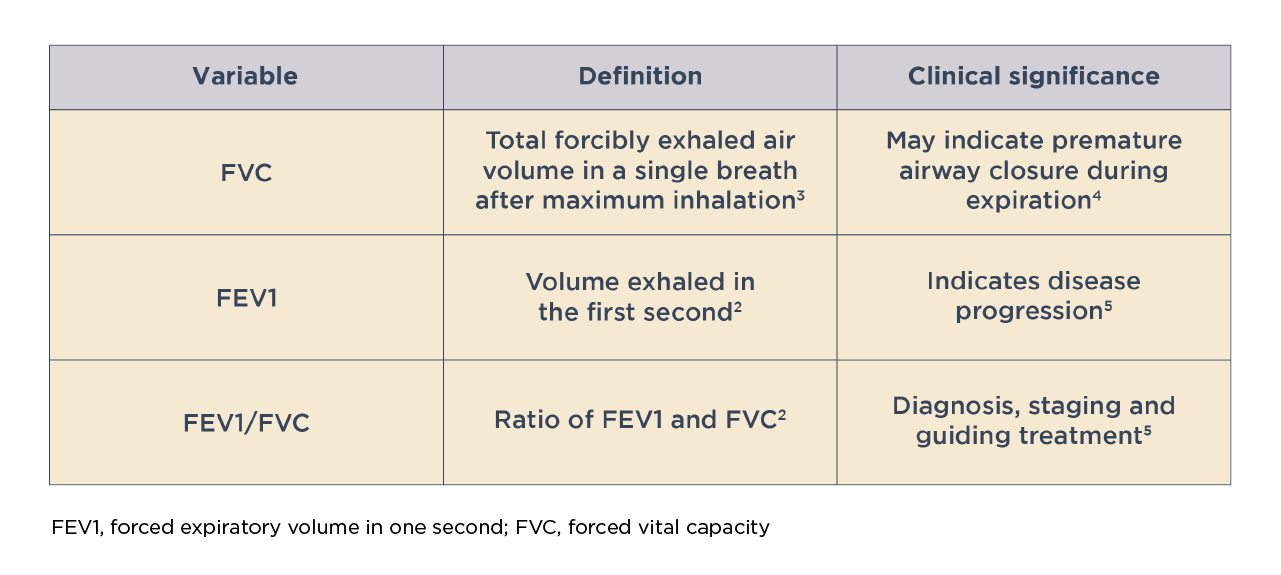
Assessment of lung function by spirometry, particularly the post-bronchodilator FEV1, plays an important role in determining COPD severity and guiding treatment.5 Possible dosage protocols for measuring post-bronchodilator FEV1 are 10-15 minutes after a short-acting beta2-agonist (dose: 400 mcg) or 30-45 minutes a short-acting anticholinergic (dose: 160 mcg) or a combination of both drug classes.1 A decline in FEV1 over time is indicative of disease progression.5
GOLD grades and severity of air flow obstruction in COPD (based on post-bronchodilator FEV1)1

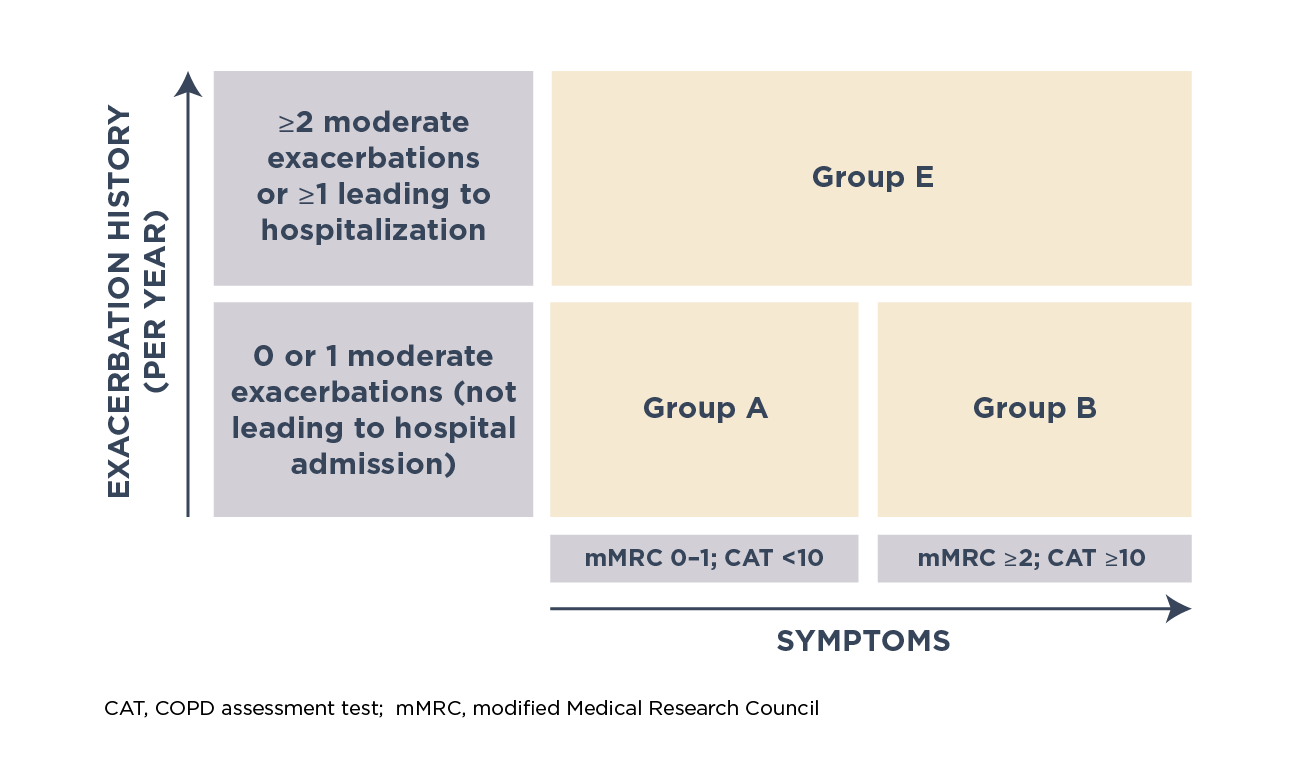
A refined GOLD ABE assessment tool, combining severity of exacerbations and symptoms, was proposed to guide pharmacological treatment. The level of symptoms is assessed based on the modified Medical Research Council (mMRC) dyspnea scale or COPD assessment test (CAT™)
Different grades of disease severity based on GOLD ABE assessment tool1
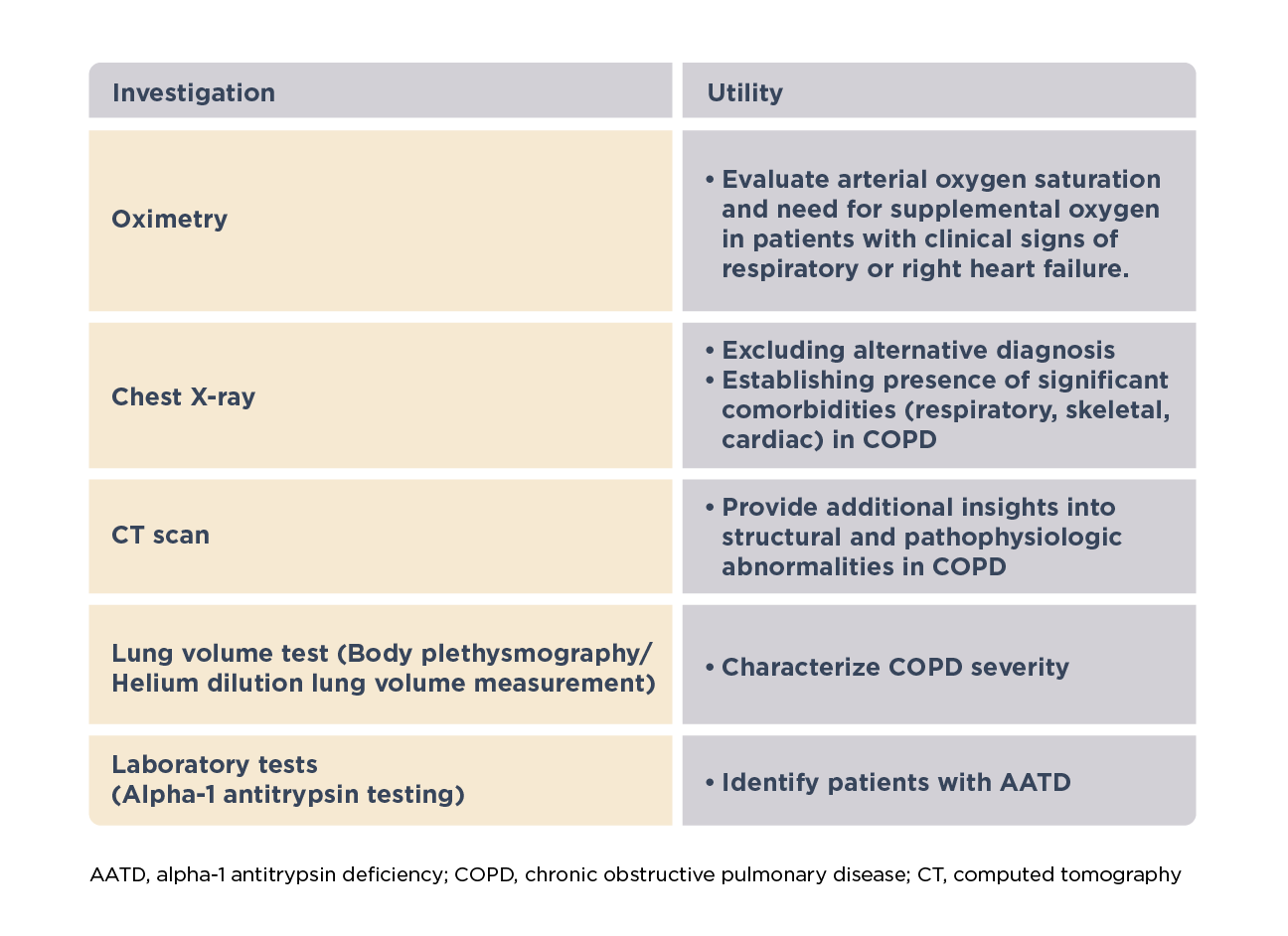
Additional clinical assessments1
Other clinical assessments may be required in specific patients to characterize severity and guide management, make a differential diagnosis, identify comorbidities or underlying other abnormalities.1
Symptom assessment using validated questionnaires
COPD impacts patient lives beyond dyspnea.1 The severity of airflow obstruction correlates weakly with symptom severity and impairment in health status.1 Therefore, changes in FEV1 are unable to capture the impact of COPD on patient’s health in its entirety.7,8 Short patient-centered questionnaires are the most efficient and accurate tools to assess severity of symptoms, limitation in activities of daily living and health-related quality of life (HRQoL).6
Understand which validated tools can be used to measure quality of life and symptoms in patients with COPD.
The implementation of quick and easy-to-use questionnaires in routine clinical practice can enable physicians fully comprehend the HRQoL of their patients, improve transparency, and prescription of appropriate treatment and effective COPD monitoring.7,8
The modified Medical Research Council (mMRC) dyspnea scale
Dyspnea is a subjective measure; therefore, it shows poor association with objective evaluation of lung function, exercise capacity, and other outcomes.5 The mMRC dyspnea scale is a simple to administer questionnaire, which assess the severity of dyspnea by grading its impact on daily activities on a scale of 0 to 4.6 Patients are required to select on a self-applied scale one of five statements that most closely describe their everyday activities or situation levels provoking breathlessness and level of impairment.5,8
COPD assessment test (CAT™)
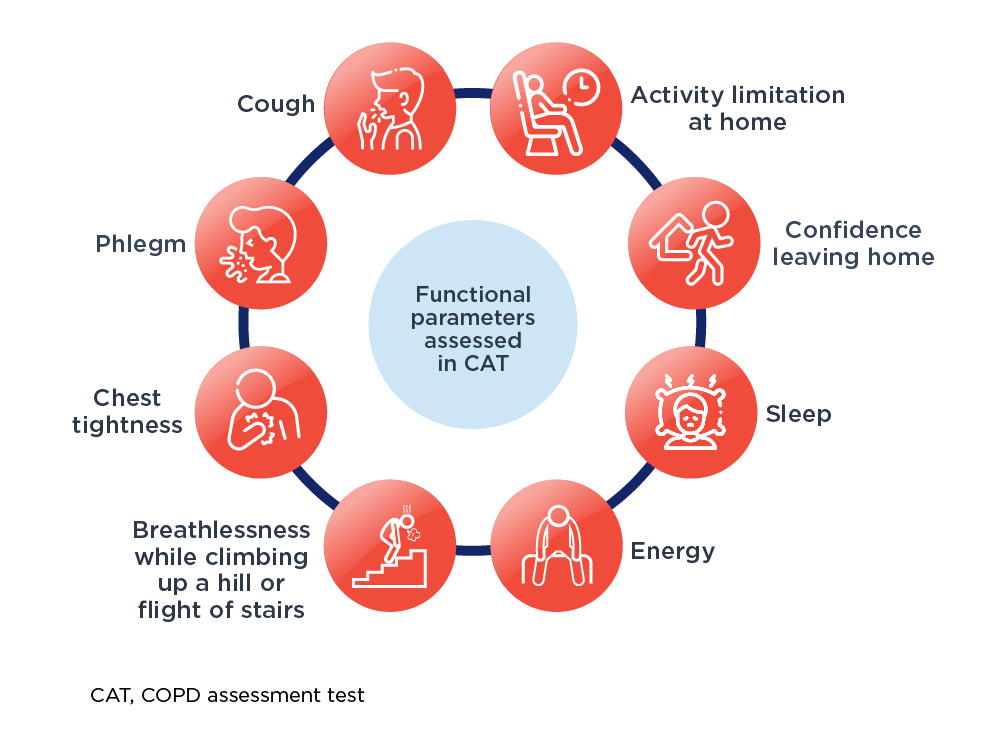
For routine use in clinical practice, the CAT is a reliable, validated, practical, simple short patient-centered questionnaire, which can quantify COPD impact on patient health.6,7 The CAT items are broad and encompass several effects of COPD on patient’s health.7 The CAT scores 8 items on a scale of 1 to 5 with a maximum total score of 40.
Read the CAT HCP user guide and FAQ to learn more about COPD assessment test
Functional parameters assessed using CAT™1,8
Blood eosinophil count
In the ECLIPSE study, 37.4% of patients with COPD had persistently raised blood eosinophil count (≥2%) over 3 years.9 When not confounded by use of inhaled corticosteroids (ICS), blood eosinophil count has a potential role as a prognostic biomarker for declining lung function.1

Blood eosinophil count can serve as a surrogate marker for sputum eosinophils to guide oral corticosteroid treatment of COPD exacerbations.9 It can help identify patients who are suitable for addition of ICS to regular bronchodilator therapy, allowing corticosteroid use in a more tailored way.1
Do you know how blood eosinophil count can help guide treatment?
Physiological test
In case of a substantial gap between perceived symptoms and severity of airflow obstruction, a better understanding of the lung physiology is required, which may require additional assessments.1 Thus, in patients with persistent symptoms after initial treatment, additional clinical assessment, including measurement of lung volumes, diffusion capacity, exercise testing and/or lung imaging may be considered. 1
References
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2024 report). Accessed [April 21, 2024]. https://goldcopd.org/wp-content/uploads/2024/02/GOLD-2024_v1.2-11Jan24_WMV.pdf.
- Lamb K, Theodore D, Bhutta BS. Spirometry. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024.
- Yawn BP. Differential assessment and management of asthma vs chronic obstructive pulmonary disease. Medscape J Med 2009;11:20.
- David S and Edwards CW. Forced Expiratory Volume. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024.
- Glaab T, Vogelmeier C, and Buhl R. Outcome measures in chronic obstructive pulmonary disease (COPD): strengths and limitations. Respir Res. 2010;11(1):79.
- Vogelmeier CF, Roman-Rodríguez M, Singh D, et al. Goals of COPD treatment: Focus on symptoms and exacerbations. Respir Med. 2020;166:105938.
- COPD assessment test. User guide. Expert guidance on frequently asked question. Available at: https://www.catestonline.org/content/dam/global/catestonline/documents/CAT_HCP%20User%20Guide.pdf . Last accessed: August 14, 2024
- van der Molen T, Miravitlles M, and Kocks JWH. COPD management: role of symptom assessment in routine clinical practice. Int J Chron Obstruct Pulmon Dis. 2013;8:461-71.
- Singh D, Kolsum U, Brightling CE, Locantore N, Agusti A, Tal-Singer R; ECLIPSE investigators. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44(6):1697-700.
- Yun JH, Lamb A, Chase R, et al. Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141(6):2037–2047.
- Bélanger M, Couillard S, Courteau J et al. Eosinophil counts in first COPD hospitalizations: a comparison of health service utilization. Int J Chron Obstruct Pulmon Dis. 2018;13:3045–3054.
- Vedel-Krogh S, Nielsen SF, Lange P et al. Blood Eosinophils and Exacerbations in Chronic Obstructive Pulmonary Disease. The Copenhagen General Population Study. Am J Respir Crit Care Med. 2016; 193:965–974.
MAT-GLB-2400917