- Article
- Source: Campus Sanofi
- Apr 1, 2024
The Impact of Exacerbations in COPD

COPD Exacerbation risk has been shown to accelerate after each exacerbation.2,a
Exacerbation risk accelerates after each exacerbation1
.webp)
*Severe exacerbations were defined as those which resulted in hospitalization with a primary discharge diagnosis of COPD2
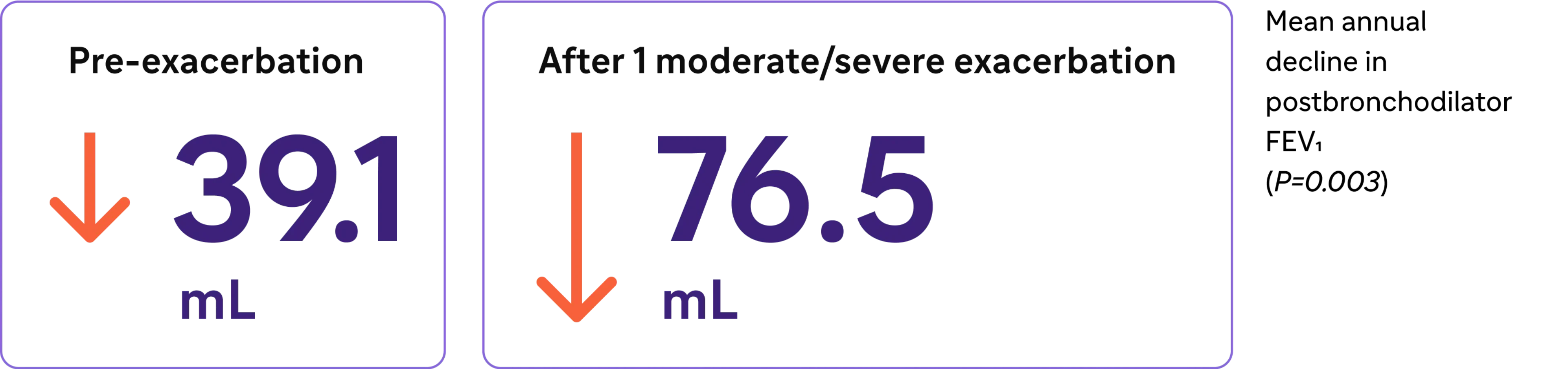
COPD Exacerbations may lead to accelerated lung function decline3,e
-
Loss of lung function nearly doubled3
-
Irreversible lung function decline may occur after only one COPD exacerbation3
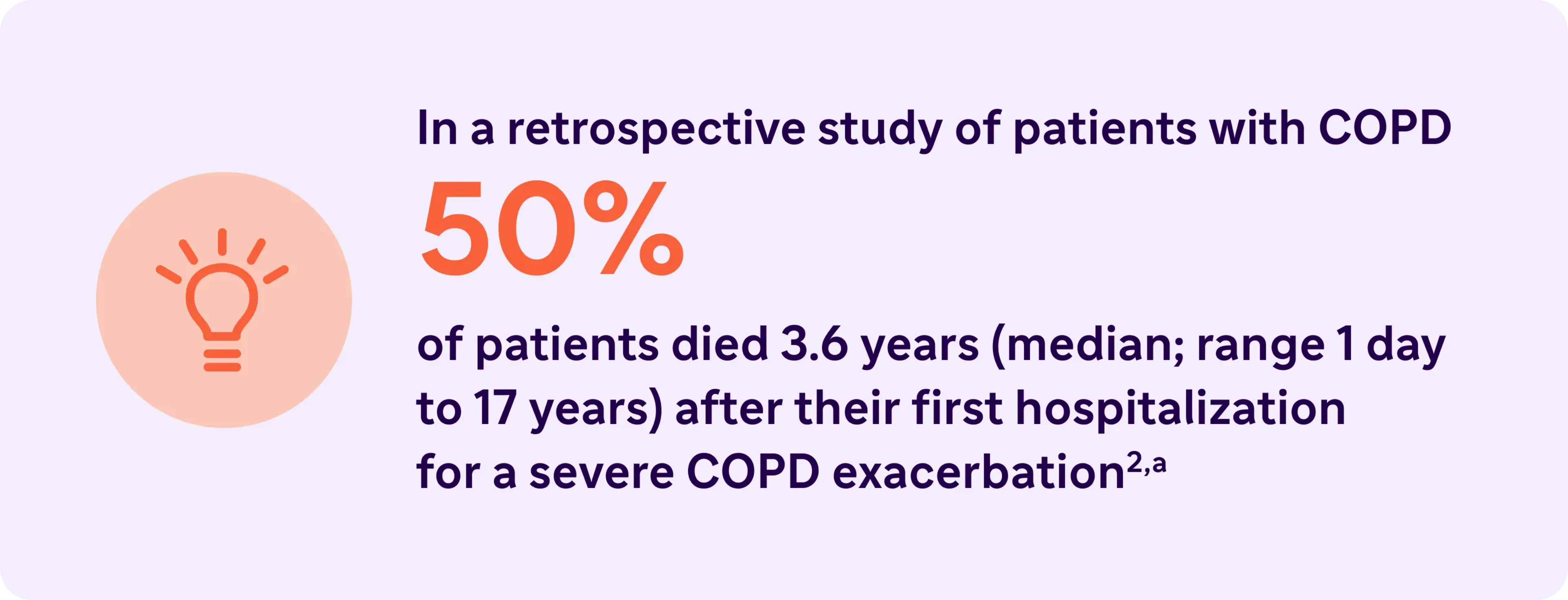
aBased on data from a large population-based cohort of 73,106 Canadian patients (mean age 75 years) who were hospitalized for the first time because of a severe exacerbation of COPD (1990-2005, followed until death or March 31, 2007).2
bAdjusted for age, sex, calendar time, and the modified Chronic Disease Score.2
aHR, adjusted hazard ratio; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; QoL, quality of life
References
1. Halpin DMG, Dransfield MT, Han MK, et al. The effect of exacerbation history on outcomes in the IMPACT trial. Eur Respir J. 2020;55:1901921. doi:10.1183/13993003.01921-2019
2. Suissa S, Dell’Anniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67(11):957-963.
3. Halpin DMG, Decramer M, Celli BR, Mueller A, Metzdorf N, Tashkin DP. Effect of a single exacerbation on decline in lung function in COPD. Respir Med. 2017;128:85-91.
4. Cosio Piqueras MG, Cosio MG. Disease of the airways in chronic obstructive pulmonary disease. Eur Respir J. 2001;18(suppl 34):41s-49s.
5. Tajti G, Gesztelyi R, Pak K, et al. Positive correlation of airway resistance and serum asymmetric dimethylarginine level in COPD patients with systemic markers of low-grade inflammation. Int J Chron Obstruct Pulmon Dis. 2017;12:873-884.
6. Higham A, Quinn AM, Cançado JED, Singh D. The pathology of small airways disease in COPD: historical aspects and future directions. Respir Res. 2019;20(1):49. doi:10.1186/s12931-019-1017-y
7. O’Donnell DE, Parker CM. COPD exacerbations. 3: Pathophysiology. Thorax. 200661(4):354-361.
8. Calverley PMA. Respiratory failure in chronic obstructive pulmonary disease. Eur Respir J. 2003;22:26s-30s.
9. Roussos C, Koutsoukou A. Respiratory failure. Eur Respir J. 2003;22(suppl 47):3s-14s.
10. Aghapour M, Raee P, Moghaddam SJ, Hiemstra PS, Heijink IH. Airway epithelial barrier dysfunction in chronic obstructive pulmonary disease: role of cigarette smoke exposure. Am J Respir Cell Mol Biol. 2018;58(2):157-169.
11. Brightling CE, Saha S, Hollins F. Interleukin-13: prospects for new treatment. Clin Exp Allergy. 2010;40(1):42-49.
12. Barberà JA, Peinado VI, Santos S. Pulmonary hypertension in chronic obstructive pulmonary disease. Eur Respir J. 2003;21(5):892-905.
13. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2024 report). Accessed [February 9, 2024]. https://goldcopd.org/2024-gold-report-2/
14. Jones PW. St George’s Respiratory Questionnaire: MCID. COPD. 2005 Mar;2(1):75-79.
15. Jones P. St George’s Respiratory Questionnaire Manual. [Version 2.4, March 2022]. Accessed [February 9, 2024]. https://www.sgul.ac.uk/research/research-operations/research-administration/st-georges-respiratory-questionnaire/docs/SGRQ-Manual-March-2022.pdf
16. Evidera website. EXACT and E-RS:COPD content. Accessed [February 9, 2024]. https://www.evidera.com/what-we-do/patient-centered-research/coa-instrument-management-services/exact-program/ exact-content/
17. Leidy NK, Bushnell DM, Thach C, Hache C, Gutzwiller FS. Interpreting Evaluating Respiratory Symptoms in COPD diary scores in clinical trials: terminology, methods, and recommendations. Chronic Obstr Pulm Dis. 2022;9(4):576-590.
18. Oshagbemi OA, Franssen FME, van Kraaij S, et al. Blood eosinophil counts, withdrawal of inhaled corticosteroids and risk of COPD exacerbations and mortality in the clinical practice research datalink (CPRD). COPD. 2019;16(2):152-159.
19. Casanova C, Celli BR, de-Torres JP, et al. Prevalence of persistent blood eosinophilia: relation to outcomes in patients with COPD. Eur Respir J. 2017;50:1701162. doi:10.1183/13993003.01162-2017
20. Singh D, Kolsum U, Brightling CE, Locantore N, Agusti A, Tal-Singer R; ECLIPSE investigators. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44(6):1697-1700.
21. Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184(6):662-671.
22. Oshagbemi OA, Burden AM, Braeken DCW, et al. Stability of blood eosinophils in patients with chronic obstructive pulmonary disease and in control subjects, and the impact of sex, age, smoking, and baseline counts. Am J Respir Crit Care Med. 2017;195(10):1402-1404.
23. Yun JH, Lamb A, Chase R, et al; COPDGene and ECLIPSE Investigators. Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141(6):2037-2047.e10. doi:10.1016/j.jaci.2018.04.010
24. Bélanger M, Couillard S, Courteau J, et al. Eosinophil counts in first COPD hospitalizations: a comparison of health service utilization. Int J Chron Obstruct Pulmon Dis. 2018;13:3045-3054.
25. Fritzsching B, Zhou-Suckow Z, Trojanek JB, et al. Hypoxic epithelial necrosis triggers neutrophilic inflammation via IL-1 receptor signaling in cystic fibrosis lung disease. Am J Respir Crit Care Med. 2015;191(8):902-913.
26. Vedel-Krogh S, Nielsen SF, Lange P, Vestbo J, Nordestgaard BG. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease. The Copenhagen General Population Study. Am J Respir Crit Care Med. 2016;193(9):965-974.
27. George L, Taylor AR, Esteve- Codina A, et al; U-BIOPRED and the EvA study teams. Blood eosinophil count and airway epithelial transcriptome relationships in COPD versus asthma. Allergy. 2020;75(2):370-380.
28. Yousuf A, Ibrahim W, Greening NJ, Brightling CE. ...for chronic obstructive pulmonary disease. J Allergy Clin Immunol Pract. 2019;7(5):1406-1416.
29. Barnes PJ. Inflammatory endotypes in COPD. Allergy. 2019;74(7):1249-1256.
30. Oishi K, Matsunaga K, Shirai T, Hirai K, Gon Y. Role of type 2 inflammatory biomarkers in chronic obstructive pulmonary disease. J Clin Med. 2020;9(8):2670. doi:10.3390/jcm9082670
31. Gabryelska A, Kuna P, Antczak A, Białasiewicz P, Panek M. IL-33 mediated inflammation in chronic respiratory diseases—understanding the role of the member of IL-1 superfamily. Front Immunol. 2019;10:692. doi:10.3389/fimmu.2019.00692
32. Allinne J, Scott G, Lim WK, et al. IL-33 blockade affects mediators of persistence and exacerbation in a model of chronic airway inflammation. J Allergy Clin Immunol. 2019;144(6):1624-1637.e10.
33. Calderon AA, Dimond C, Choy DF, et al. Targeting interleukin-33 and thymic stromal lymphopoietin pathways for novel pulmonary therapeutics in asthma and COPD. Eur Respir Rev. 2023;32(167):220144. doi:10.1183/16000617.0144-2022
34. Gandhi NA, Bennett BL, Graham NMH, Pirozzi G, Stahl N, Yancopoulos D. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016;15(1):35-50.
35. Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007;119(6):1303-1310.
36. Doyle AD, Mukherjee M, LeSuer WE, et al. Eosinophil-derived ... promotes emphysema. Eur Respir J. 2019;53(5):1801291. doi:10.1183/13993003.01291-2018
37. Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138(1):16-27.
38. Defrance T, Carayon P, Billian G, et al. ... is a B cell stimulating factor. J Exp Med. 1994;179(1):135-143.
39. Yanagihara Y, Ikizawa K, Kajiwara K, Koshio T, Basaki Y, Akiyama K. Functional significance of ... receptor on B cells in ... – induced human IgE production. J Allergy Clin Immunol. 1995;96(6 pt 2):1145-1151.
40. Gandhi NA, Pirozzi G, Graham NMH. Commonality of the ... pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13(5):425-437.
41. Kaur D, Hollins F, Woodman L, et al. Mast cells express ... promotes human lung mast cell proliferation and FcεRI expression. Allergy. 2006;61(9):1047-1053.
42. Saatian B, Rezaee F, Desando S, et al. ... cause barrier dysfunction in human epithelial cells. Tissue Barriers. 2013;1(2):e24333. doi:10.4161/tisb.24333
43. Zheng T, Zhu Z, Wang Z, et al. Inducible targeting of ... to the adult lung causes matrix metalloproteinase– and cathepsin-dependent emphysema. J Clin Invest. 2000;106(9):1081-1093.
44. Garudadri S, Woodruff PG. Targeting chronic obstructive pulmonary disease phenotypes, endotypes, and biomarkers. Ann Am Thorac Soc. 2018;15(suppl 4):S234-S238.
45. Alevy YG, Patel AC, Romero AG, et al. ... induced airway mucus production is attenuated by MAPK13 inhibition. J Clin Invest. 2012;122(12):4555-4568.
46. Singanayagam A, Footitt J, Marczynski M, et al. Airway mucins promote immunopathology in virus-exacerbated chronic obstructive pulmonary disease. J Clin Invest. 2022;132(8):e12901. doi:10.1172/JCI120901
47. Zhu Z, Homer RJ, Wang Z, et al. Pulmonary expression of ... causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103(6):779-788.
48. Cooper PR, Poll CT, Barnes PJ, Sturton RG. Involvement of ... in tobacco smoke-induced changes in the structure and function of rat intrapulmonary airways. Am J Respir Cell Mol Biol. 2010;43(2):220-226.
49. Arora S, Dev K, Agarwal B, Das P, Syed MA. Macrophages: their role, activation, and polarization in pulmonary diseases. Immunobiology. 2018;223(4-5):383-396.
50. He S, Xie L, Lu J, Sun S. Characteristics and potential role of M2 macrophages in COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:3029-3039.
51. Wang X, Xu C, Ji J, et al. ... upregulates Sonic hedgehog expression to induce allergic airway epithelial remodeling. Am J Physiol Lung Cell Mol Physiol. 2020;318(5):L888-L899.
52. Linden D, Guo-Parke H, Coyle PV, et al. Respiratory viral infection: a potential “missing link” in the pathogenesis of COPD. Eur Respir Rev. 2019;28(151):180063. doi:10.1183/16000617.0063-2018
53. Wang Z, Bafadhel M, Haldar K, et al. Lung microbiome dynamics in COPD exacerbations. Eur Respir J. 2016;47(4):1082-1092.
MAT-GLB-2400917