- Article
- Source: Campus Sanofi
- Jul 4, 2024
GOLD Recommendations for Treatment and Management of COPD


The GOLD COPD report
The GOLD COPD report offers non-biased review and recommendation for diagnosis, management, and treatment of patients with COPD, categorized by disease severity.1
The objectives of the GOLD COPD treatment report are:1
- Relieving and reducing the impact of symptoms
- Reducing the risk of adverse health events (e.g., exacerbation)
Clinicians need to focus on short-term and long-term impact of COPD on their patients.1
-
- To learn more about the COPD GOLD report, read this article Global Initiative for Chronic Obstructive Lung Disease
Classification of COPD based on GOLD report
As per the GOLD COPD report, pulmonary function lung testing involves measuring the ratio of forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC). A post-bronchodilator FEV1/FVC ratio <0.70 is commonly considered a diagnostic criterion for COPD.1 The FEV1/FVC ratio helps in accurate and repeatable measurement of lung function as recommended by GOLD for COPD diagnosis.
The GOLD recommendations for treatment of patients with COPD is based on airflow limitation categories:1
- GOLD 1 (Mild): FEV1 ≥80% predicted
- GOLD 2 (Moderate): 50% ≤FEV1 <80% predicted
- GOLD 3 (Severe): 30% ≤FEV1 <50% predicted
- GOLD 4 (Very Severe): FEV1 <30% predicted
The GOLD ABE assessment Tool
The GOLD ABE assessment tool has been developed to provide a comprehensive evaluation of patients with COPD. This tool incorporates patients’ symptomatic assessment with spirometric classification, and/or the risk of exacerbation, to guide initial pharmacological treatment.1 The GOLD COPD report recommends that patients should:
- Undergo spirometry to determine the severity of airflow limitation.1
- Undergo an assessment of their dyspnea using the modified Medical Research Council (mMRC) dyspnea scale or symptoms using the COPD Assessment Test (CAT).1
- Have their history of moderate or severe exacerbation (including prior hospitalization) recorded.1
○ Link to key tools to measure the success of COPD management

GOLD ABE categories:
The previously used A, B, C, and D categories in the assessment tool recommended by GOLD COPD report were revised to A, B, and E categories to highlight clinical relevance of previous exacerbations.1 The categories are defined by considering two specific features:1
- Exacerbation history: This includes the patient’s history of moderate or severe exacerbations, including any prior hospitalizations.
- Symptom score: This can be assessed using either the mMRC dyspnea scale or the COPD Assessment Test (CAT).
Please refer below figure for more details:
GOLD COPD ABE assessment tool1
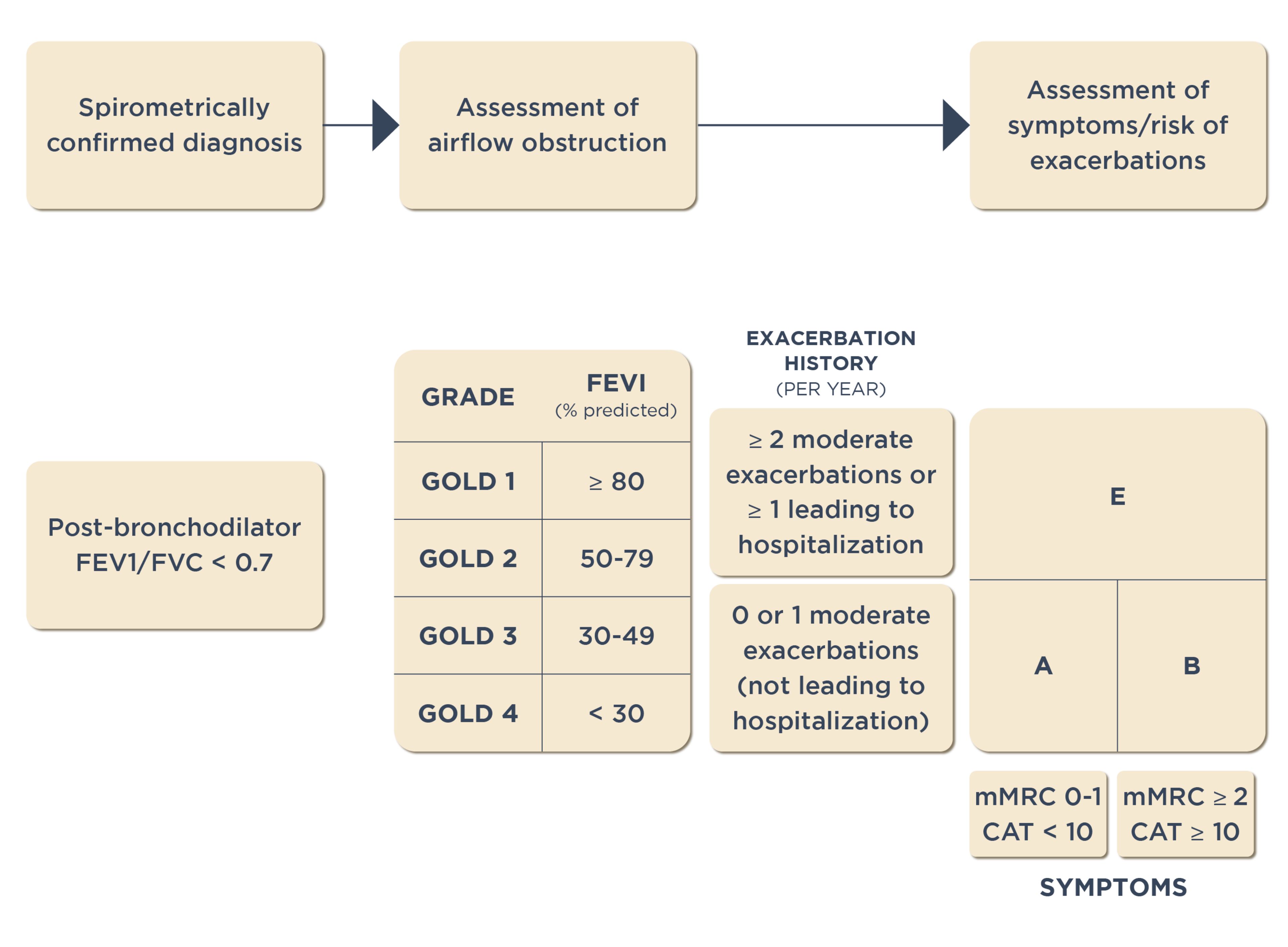
It is critical for healthcare professionals to categorize patients appropriately as per the GOLD ABE assessment tool and explain the right classification to their patients to ensure they understand their condition and the appropriate treatment plan.
GOLD COPD treatment recommendations
Pharmacological therapy for COPD is used to reduce symptoms, the frequency and severity of exacerbations, and improve exercise tolerance and health status. GOLD COPD treatment report recommends initiating treatment based on the level of symptoms and risk for exacerbations, and that initial pharmacotherapy should be based on the patient’s GOLD severity group.1
- To learn more about the GOLD COPD report, read this article Global Initiative for Chronic Obstructive Lung Disease
Algorithm for the initiation and monitoring of pharmacological treatment:
Guidance for the pharmacological treatment of COPD is based on the individualized assessment of symptoms and exacerbation risk following the ABE scheme, and accounting for blood eosinophil count.1 The GOLD COPD recommendations for treatment by ABE group is distinct:
- Group A: A short-acting or long-acting bronchodilator as needed
- Group B: Long-acting bronchodilator (LABA + LAMA)
- Group E: LABA + LAMA or LABA + LAMA + inhaled corticosteroids (ICS) if blood eosinophils ≥300 cells/µL
Algorithm of initial pharmacological treatment of patients with COPD1

Latest GOLD report for COPD: Considering eosinophil counts
Higher blood eosinophil counts in COPD patients are associated with the presence of higher levels of markers of type-2 inflammation in the airways. GOLD recommends that blood eosinophil count should be used to guide the use of ICS as part of COPD management. Eosinophil counts can help clinicians predict if a patient is more likely to respond well to the addition of ICS to regular bronchodilator treatment, making it a valuable biomarker alongside clinical assessment when making decisions regarding ICS use.
Blood eosinophil counts could also be used as a prognostic biomarker for lung function decline when not confounded by ICS use. In younger individuals without COPD, raised blood eosinophil counts are associated with increased risk of subsequent development of COPD.1
Follow-up pharmacological management:1
Treatment recommendations in the GOLD COPD report are intended to be tailored to individual patient needs and responses. Regular follow-up and reassessment are essential to ensure optimal management of COPD. The follow-up treatment is adjusted based on the patient’s response to initial treatment and their exacerbation history. Please refer the below pages for more information on pharmacological management
-
- To learn more about the GOLD COPD report, read this article Global Initiative for Chronic Obstructive Lung Disease

GOLD recommendations for the non-pharmacological treatment of COPD
Non-pharmacological strategies recommended by GOLD for COPD treatment are complementary to the pharmacological treatment regimen.1 Non-pharmacologic treatments for COPD depend on the patient's GOLD classification (A, B, or E) and include smoking cessation, pulmonary rehabilitation, vaccinations, and oxygen therapy when indicated.1
Smoking cessation:
According to GOLD COPD report smoking cessation is a key intervention for patients with COPD who continue to smoke. It has the capacity to influence the natural history of COPD, improve daily symptoms, and decrease the frequency of exacerbations.1 Five recommended strategies and support methods as per the GOLD COPD report to help the patients willing to quit smoking are illustrated below.1
Strategies to help patients willing to quit smoking1
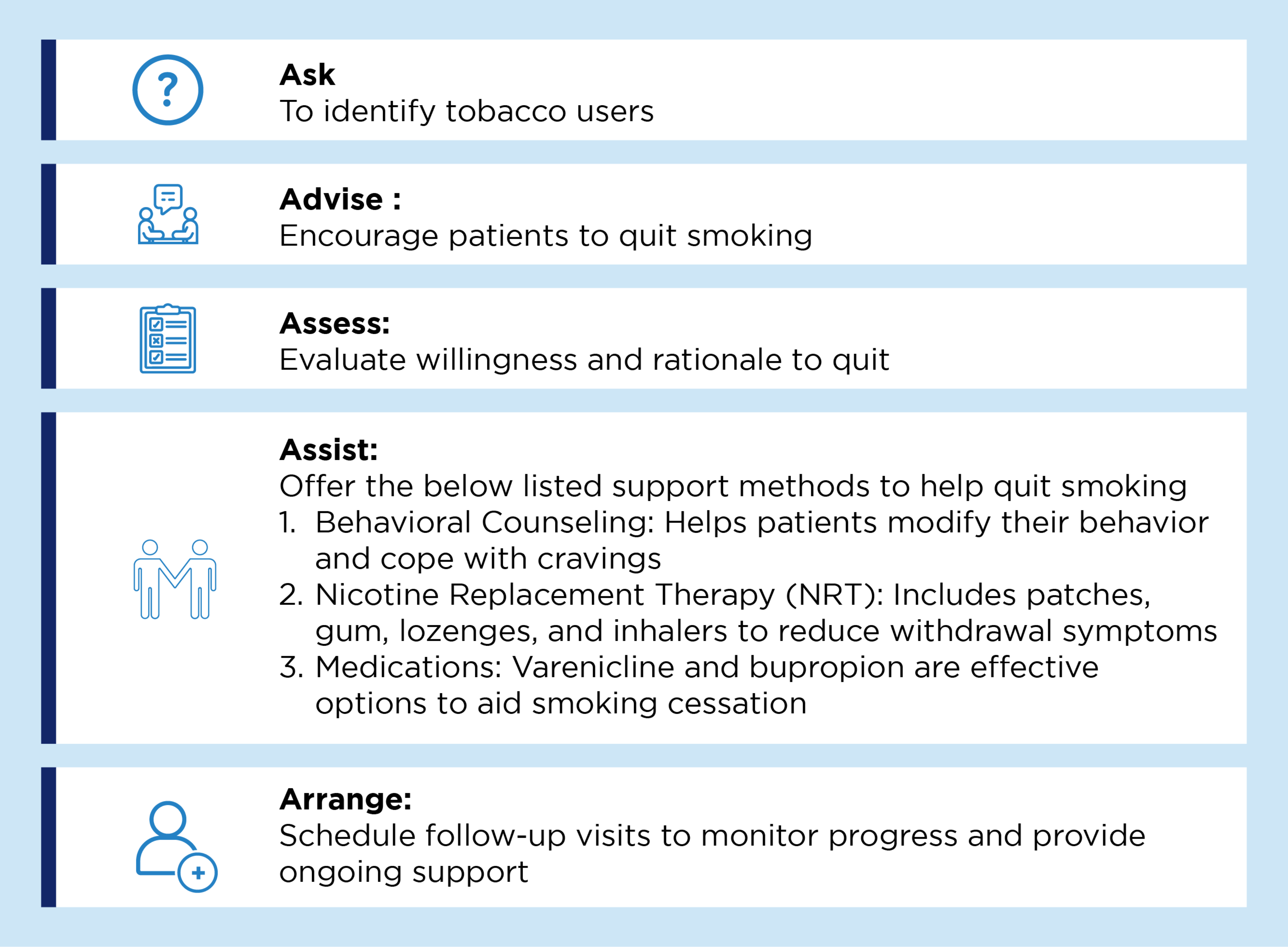
Pulmonary rehabilitation:
Pulmonary rehabilitation involves but is not limited to exercise training with disease-specific education aimed at improving the physical and psychological condition of patients. Patients with high symptom burden and risk of exacerbations, should be encouraged to take part in a formal rehabilitation program.1
Key updates in 2024 GOLD COPD report1,2
Key changes in the latest report include an update on blood eosinophil count based treatment recommendations in the initial assessment section and revisions to smoking cessation section under prevention and management of COPD. Please refer to the 2024 GOLD COPD report for additional updates.1,2
COPD GOLD Pocket Guide and PDF:
- To learn more about the GOLD COPD report, please refer to 2024 GOLD Pocket Guide and full PDF
References
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2024 report). Accessed [June 10, 2024]. https://goldcopd.org/wp-content/uploads/2024/02/GOLD-2024_v1.2-11Jan24_WMV.pdf.
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2023 report). Accessed [June 10, 2024]. https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf.
- Yun JH, Lamb A, Chase R, et al. Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141(6):2037–2047.
MAT-SA-2400418/V1/SEP24