- Article
- Source: Campus Sanofi
- May 23, 2025
Understanding the Changes in the GOLD Report from 2024 to 2025

The Global Initiative for Chronic Obstructive Lung Disease (GOLD) report is an essential resource for healthcare professionals (HCPs) involved in the diagnosis, management, and prevention of Chronic Obstructive Pulmonary Disease (COPD). The 2025 update introduces significant changes aimed at enhancing patient outcomes through refined diagnostic criteria and treatment strategies. This article delves into these key changes, emphasizing their impact on HCP approaches to screening and managing patients with COPD.
Overview of GOLD COPD Report: 2025 update
The GOLD report, a comprehensive guide developed by the GOLD Science Committee, provides a non-biased review of current evidence for the assessment, diagnosis, and treatment of COPD. The GOLD report is revised on an annual basis with the primary objective of aiding clinicians in delivering the best possible care to patients with COPD.1 The 2025 update continues this tradition by incorporating the latest research findings and clinical practices to improve patient outcomes.
The 2025 GOLD report introduces several noteworthy changes in the diagnosis and treatment of COPD.1 The following key sections were revised:
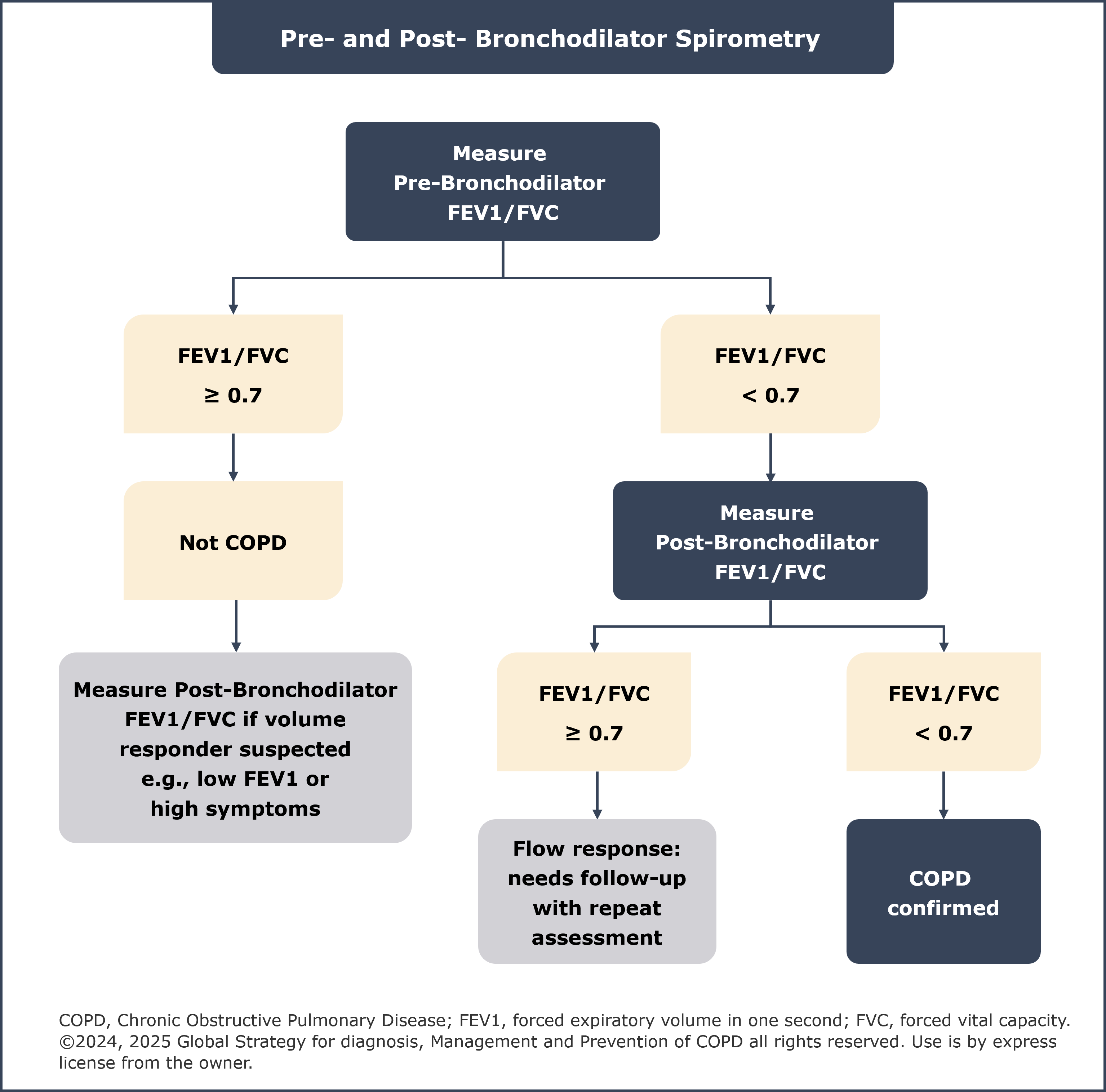
1. Updated spirometry section
A new flowchart elaborating on the role of pre- and post-bronchodilator spirometry for the assessment of airflow obstruction and diagnosis of COPD has been included in the spirometry section. For diagnosis of COPD, a forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ratio of <0·7 on post-bronchodilator spirometry still remains mandatory. Consistent with the 2024 GOLD report,2 it is not recommended to measure FEV1 before and after bronchodilators or corticosteroids for assessing degree of reversibility and guiding therapeutic decisions.1
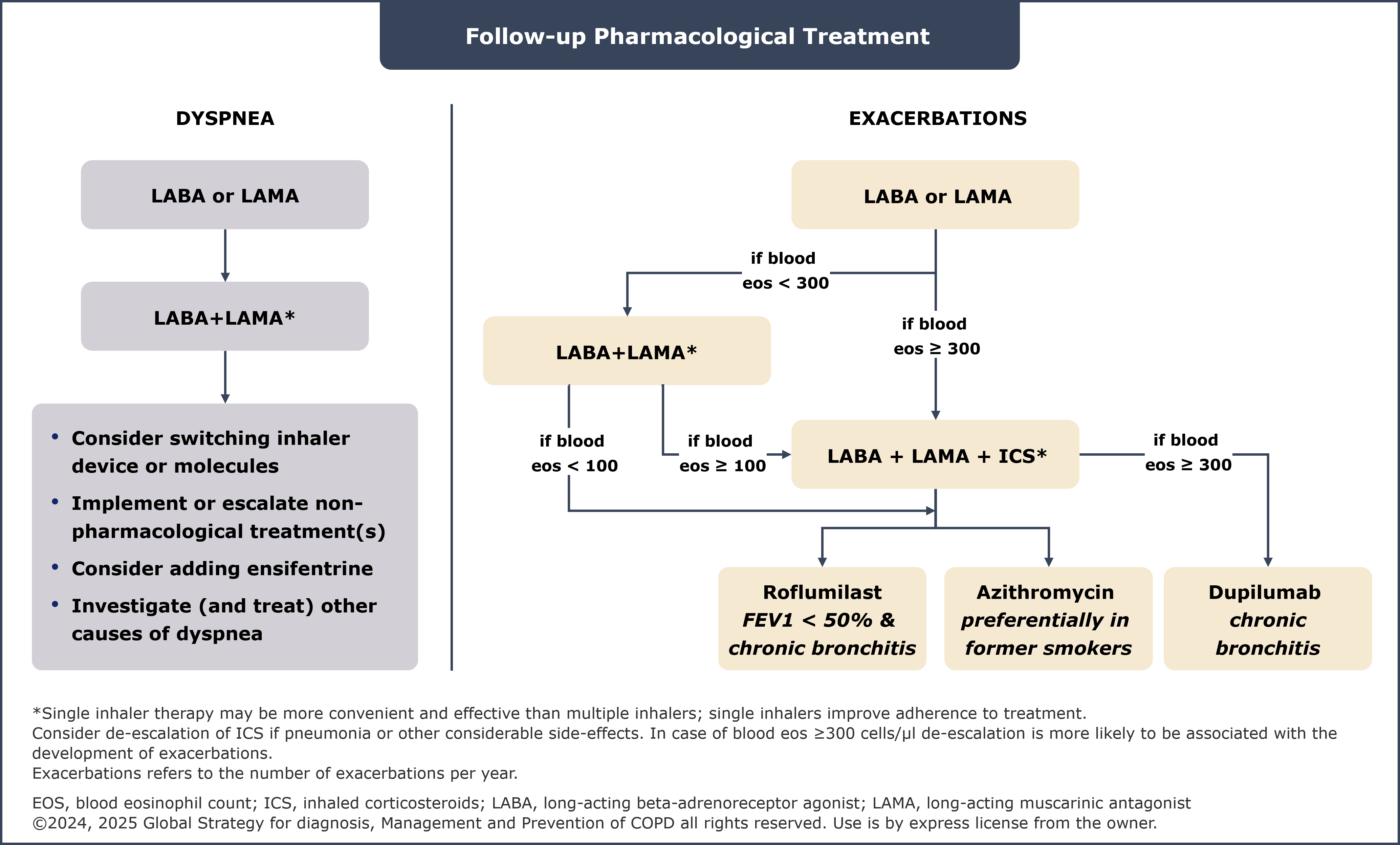
Updated follow-up pharmacological management strategies
The “Algorithms for follow-up pharmacological management” section has been updated to include new treatment options for patients with dyspnea and persistent exacerbations and provide clear guidance on management of patients currently on long-acting beta-adrenoreceptor agonist (LABA) + inhaled corticosteroid (ICS).
Dyspnea
If addition of a second long-acting bronchodilator does not improve symptoms in patients with persistent breathlessness or exercise limitation on bronchodilator monotherapy, the 2024 GOLD report recommended considering switching inhaler device or pharmacotherapy. The 2025 GOLD report additionally recommends the following:
- Implementing or escalating non-pharmacological treatment(s) e.g., pulmonary rehabilitation
- Considering adding ensifentrine, if available
Persistent exacerbations
In patients treated with LABA + long-acting muscarinic antagonist (LAMA) and blood eosinophil count (EOS) <100 cells/µL who still have exacerbations, the following treatment is recommended1:
- Among those who are not currently smoking, consider adding azithromycin. Consideration to the development of resistant organisms should be factored into decision making
- Among those with FEV1 <50%, symptoms of chronic bronchitis and history of prior severe exacerbation, consider adding roflumilast
In patients treated with LABA+LAMA+ICS (or those with EOS <100 cells/µL) who still have exacerbations, the following treatment is recommended1:
- Among those who are not currently smoking, consider adding azithromycin. Consideration to the development of resistant organisms should be factored into decision making
- Among those with FEV1 <50%, symptoms of chronic bronchitis and history of prior severe exacerbation, consider adding roflumilast
- Among those with EOS ≥300 cells/µL and symptoms of chronic bronchitis, consider adding dupilumab
To learn about the stability and predictive value of blood EOS in COPD, please click here.
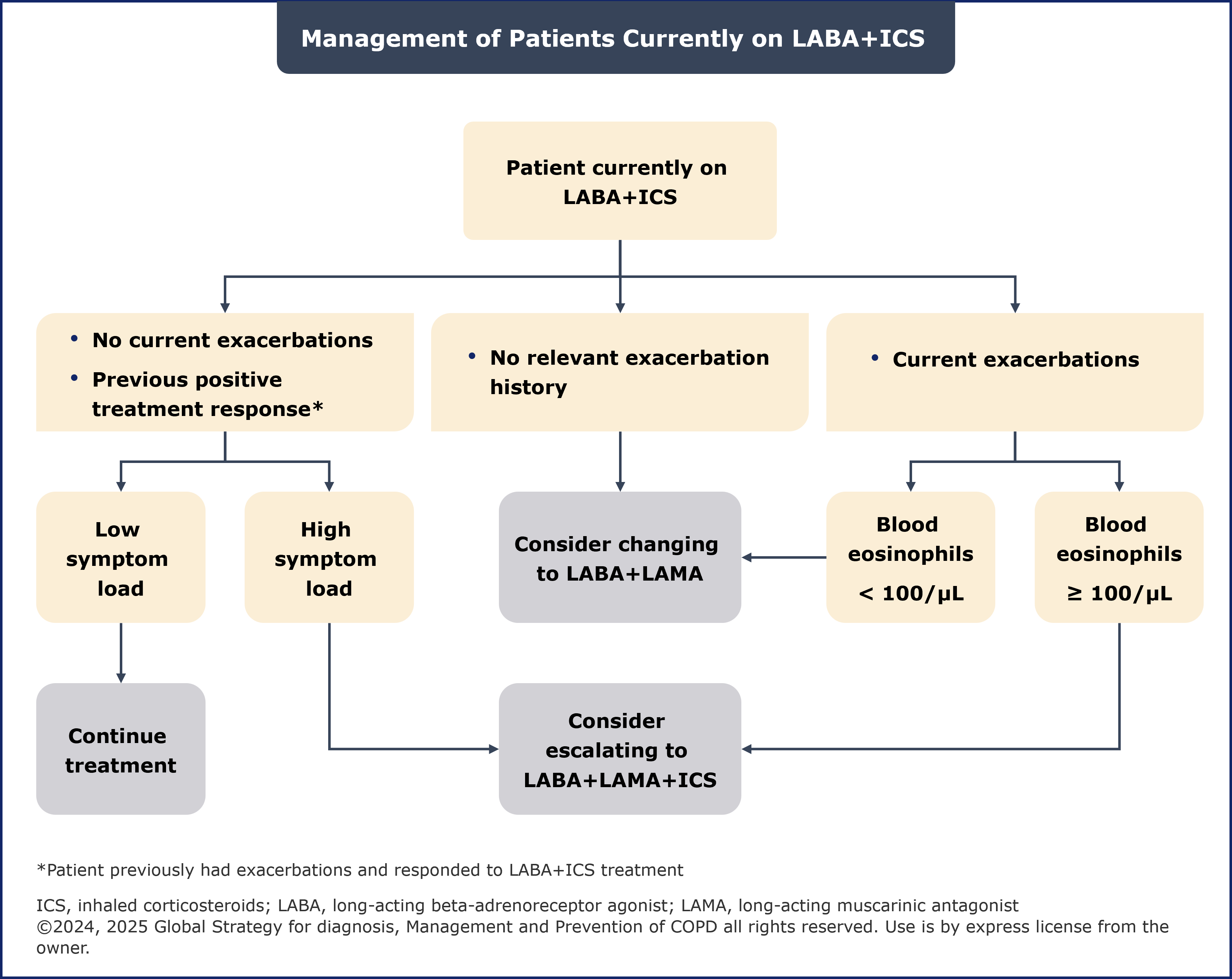
Management of patients currently on LABA+ICS
Generally, if there is an indication for ICS use then LABA+LAMA+ICS has been shown to be superior to LABA+ICS. For patients on LABA+ICS with major symptoms, the 2024 report recommended considering a switch to LABA+LAMA.2 The 2025 report recommends changing to LABA+LAMA or LABA+LAMA+ICS while considering previous response to ICS treatment.1
The benefits and risks of ICS withdrawal should be carefully considered, with a blood EOS count >300 cells/µL being an indicator of increased risk of exacerbations with ICS withdrawal.1 Additionally, the updated 2025 report emphasizes that the ICS component should not be withdrawn in patients treated with LABA+LAMA+ICS unless the ICS were started inappropriately or there has been no response to ICS, or patients experience significant side-effects or severe or recurrent pneumonia.1
Furthermore, it is important to review whether there was a relevant prior exacerbation history or a previous positive response to LABA+ICS treatment. A history of exacerbations but absence of current exacerbations suggests a positive response to ICS treatment.1
2. Introduction of new pharmacotherapies
The 2025 report included two new drugs in the list of pharmacotherapies, a phosphodiesterase 3 and 4 (PDE 3 and PDE 4) inhibitor and a biologic that blocks the shared receptor component of IL-4 and IL-13.
Phosphodiesterase 3 and 4 (PDE 3 and PDE 4) inhibitor
The PDE 3 and PDE 4 inhibitor, delivered via nebulizer, is now included as a maintenance medication and bronchodilator for stable COPD. It is known to significantly improve lung function, dyspnea and health status.1 The drug has shown significant improvement in lung function and dyspnea with no safety or tolerability issues,3 in parallel phase 3 studies.4
Drugs with potential to reduce exacerbations
This section has added evidence to support the efficacy of the biologic therapy, a fully human monoclonal antibody that blocks the shared receptor component of IL-4 and IL-13. The biologic was evaluated in a study population comprising COPD patients with symptoms of chronic bronchitis, a history of ≥2 moderate exacerbations or ≥1 severe exacerbation(s) in the previous year despite treatment with LABA+LAMA+ICS, and blood EOS count ≥300 cells/µL.1 Patients receiving this biologic demonstrated fewer exacerbations, better lung function and improved symptoms over 52 weeks in two large, double-blind, randomized phase 3 studies.5,6
Summary of the key Updates and Why They Matter
The changes introduced in the 2025 GOLD report aim to enhance the accuracy of diagnosis of COPD and latest treatment strategies. By refining the sections on spirometry, follow-up pharmacological treatment, management of patients and reflecting on the latest evidence on newly recommended biologic therapy with the potential to reduce exacerbations, the report provides a comprehensive framework for improving patient outcomes.
The revised follow-up pharmacological management strategies suggested escalation and de-escalation based on available efficacy and safety data.1 For healthcare professionals, these changes along with the revised algorithms for pharmacological treatment means adopting a more evidence-based and personalized approach to COPD management.
Know more about the role of Blood Eosinophils as a Biomarker in Patients with COPD
In conclusion, the 2025 GOLD report represents a significant step forward in the diagnosis and management of COPD. By staying informed about these changes and incorporating them into clinical practice, healthcare professionals can continue to provide the highest standard of care to their patients.
Click here to refer to the GOLD 2025 report for more information.
References
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2025 report) (Accessed November 28, 2024, at https://goldcopd.org/2025-gold-report/)
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2024 report) (Accessed December 4, 2024, at https://goldcopd.org/2024-gold-report/)
- Singh D, Lea S and Mathioudakis AG. Inhaled Phosphodiesterase Inhibitors for the Treatment of Chronic Obstructive Pulmonary Disease. Drugs. 2021 Nov;81(16):1821-1830. doi: 10.1007/s40265-021-01616-9. Epub 2021 Nov 3. PMID: 34731461.
- Anzueto A, Barjaktarevic IZ, Siler TM, Rheault T, Bengtsson T, Rickard K, et al. Ensifentrine, a Novel Phosphodiesterase 3 and 4 Inhibitor for the Treatment of Chronic Obstructive Pulmonary Disease: Randomized, Double-Blind, Placebo-controlled, Multicenter Phase III Trials (the ENHANCE Trials). Am J Respir Crit Care Med. 2023 Aug 15;208(4):406-416. doi: 10.1164/rccm.202306-0944OC. PMID: 37364283; PMCID: PMC10449067.
- Bhatt SP, Rabe KF, Hanania NA, Vogelmeier CF, Cole J, Bafadhel M, et al. BOREAS Investigators. Dupilumab for COPD with Type 2 Inflammation Indicated by Eosinophil Counts. N Engl J Med. 2023 Jul 20;389(3):205-214. doi: 10.1056/NEJMoa2303951. Epub 2023 May 21. PMID: 37272521.
- Bhatt SP, Rabe KF, Hanania NA, Vogelmeier CF, Bafadhel M, Christenson SA, et al.NOTUS Study Investigators. Dupilumab for COPD with Blood Eosinophil Evidence of Type 2 Inflammation. N Engl J Med. 2024 Jun 27;390(24):2274-2283. doi: 10.1056/NEJMoa2401304. Epub 2024 May 20. PMID: 38767614.
MAT-GLB-2400917