- Article
- Source: Campus Sanofi
- Aug 26, 2024
How should individuals with positive autoimmune T1D autoantibodies be monitored over time?

Key Takeaway
* Consensus report endorsed by EASD, ADA, AACE, ACD, ADCES, ADS, ISPAD, ATTD, DiaUnion, the Endocrine Society and JDRF International.
What are the next steps for individuals who test positive for T1D autoantibodies?
A positive screening for presence of ≥2 autoimmune T1D antibodies indicate that the individual is in presymptomatic stage of autoimmune T1D.2 As per the latest consensus guidance, such individuals need metabolic monitoring for the presence of glucose abnormalities, an indicator of disease progression.1,3,4
Presence of dysglycemia, (fasting plasma glucose ≥100 mg/dL [≥5.6 mmol/L], 2-hour plasma glucose with 75 gm OGTT ≥140 mg/dL [≥7.8 mmol/L], 30/60/90 min glucose ≥200 mg/dL [≥11.1 mmol/L], and/or HbA1c ≥5.7% [≥39 mmol/mol]), in individuals who test positive for ≥2 autoimmune T1D antibodies indicate Stage 2 autoimmune T1D.3 Individuals in this stage have a 100% risk of progressing to symptomatic Stage 3 autoimmune T1D within the next 15 years.2 At this stage (Stage 2) you can provide such individuals and their caregivers the relevant disease-related education and psychosocial support needed to prepare for a possible Stage 3 autoimmune T1D diagnosis. 4,5
What is the purpose of monitoring T1D autoantibodies and glycemia?
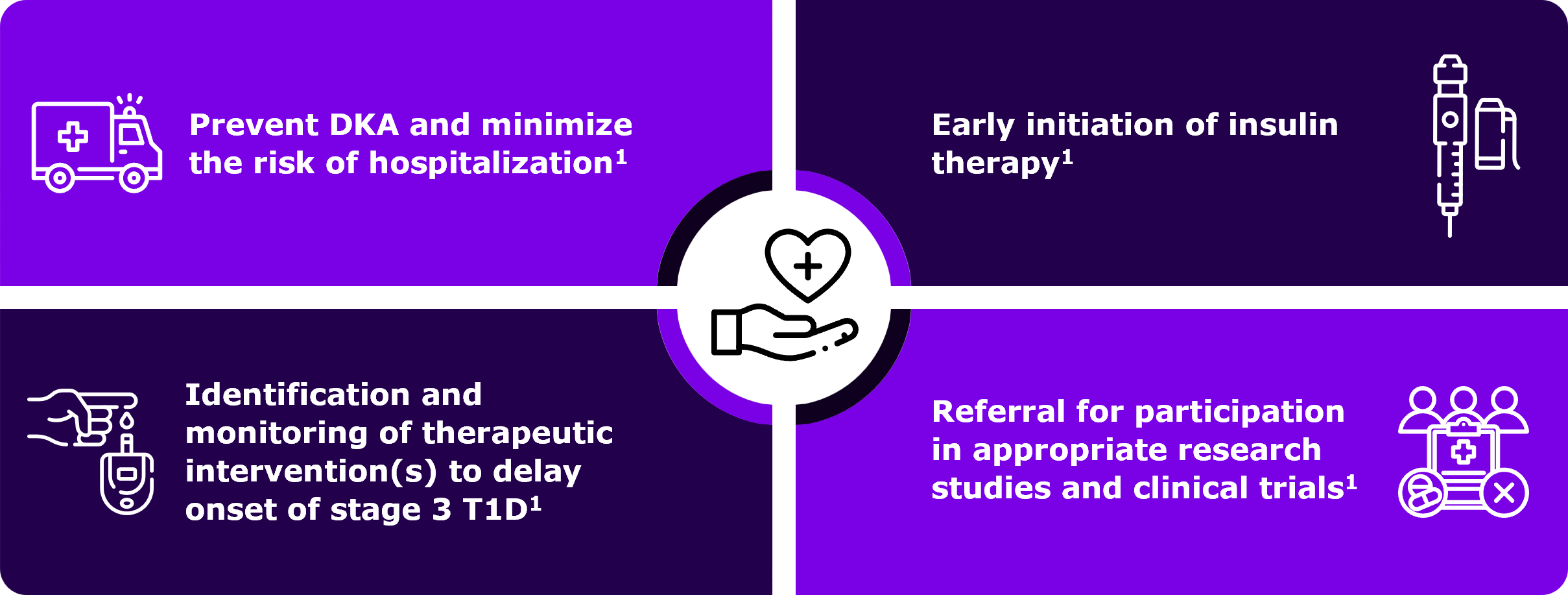
The primary purpose of monitoring individuals with positive screening results for autoimmune T1D antibodies is to potentially lower the risk of diabetic ketoacidosis (DKA) at Stage 3 autoimmune T1D diagnosis.1 DKA is a life-threatening condition which is associated with need for hospital admission and potential long-term complications.1 A lower risk of DKA is associated with lower incidence of severe hypoglycemic events within 10 years post diagnosis.1 Regular medical monitoring, including monitoring glucose abnormalities, will help identify disease progression early on.1,4
Timely monitoring that helps in the early detection of Stage 3 autoimmune T1D can enable you to promptly initiate early insulin therapy which will help to optimize HbA1c and potentially avoid long-term consequences of prolonged hyperglycemia.1 Monitoring further avoids misdiagnosis of type 2 diabetes and delay in treatment, and also it may increase patient referrals to clinical trials where appropriate.1
Should you follow specific algorithms and tools to monitor individuals who test positive for T1D autoantibodies?
Monitoring of individuals who screened positive for islet autoantibodies can be largely divided into the two categories:
- Repeat antibody testing: Once an individual tests positive, the second confirmatory test should be done within 3 months.1 A persistent presence of >1 autoantibody indicates presymptomatic Stage 1 autoimmune T1D.1 Repeat periodic assessments confirming presence of ≥2 autoantibodies along with onset of dysglycemia indicates progression of disease to Stage 2 autoimmune T1D.1
- Metabolic monitoring: Choose from multiple available tools, such as regular oral glucose tolerance test (OGTT), random venous or capillary blood glucose, continuous glucose monitoring (CGM), HbA1c testing or self-monitoring of blood glucose (SMBG).1 Testing C-peptide can help you assess measure of beta-cell function and distinguish stages of T1D.1
A detailed description of the methods used for metabolic monitoring is provided in the table below.
| Metric |
Pros | Cons | Information gained |
| Oral glucose tolerance test (OGTT)4 |
|
|
|
| Random venous glucose4,6 |
|
|
|
| HbA1c4 |
|
|
|
| Continuous Glucose monitoring (CGM) |
|
|
|
| Self monitoring blood glucose (SMBG)4 |
|
|
|
At any point of monitoring if you observe onset of hyperglycemia along with other clinical symptoms such as polyuria, polydipsia, weight loss, fatigue, hunger, blurry vision, and diabetic ketoacidosis (DKA), it indicates disease progression to symptomatic Stage 3 autoimmune T1D.1
The consensus guidance published in June 2024 provides a detailed algorithm for monitoring of individuals who have been screened positive for islet autoantibodies.1 Below figure illustrates an algorithm for monitoring of people screened positive for ≥2 islet autoantibodies.1
Decision path for monitoring of individuals screened positive for ≥1 islet autoantibodies1
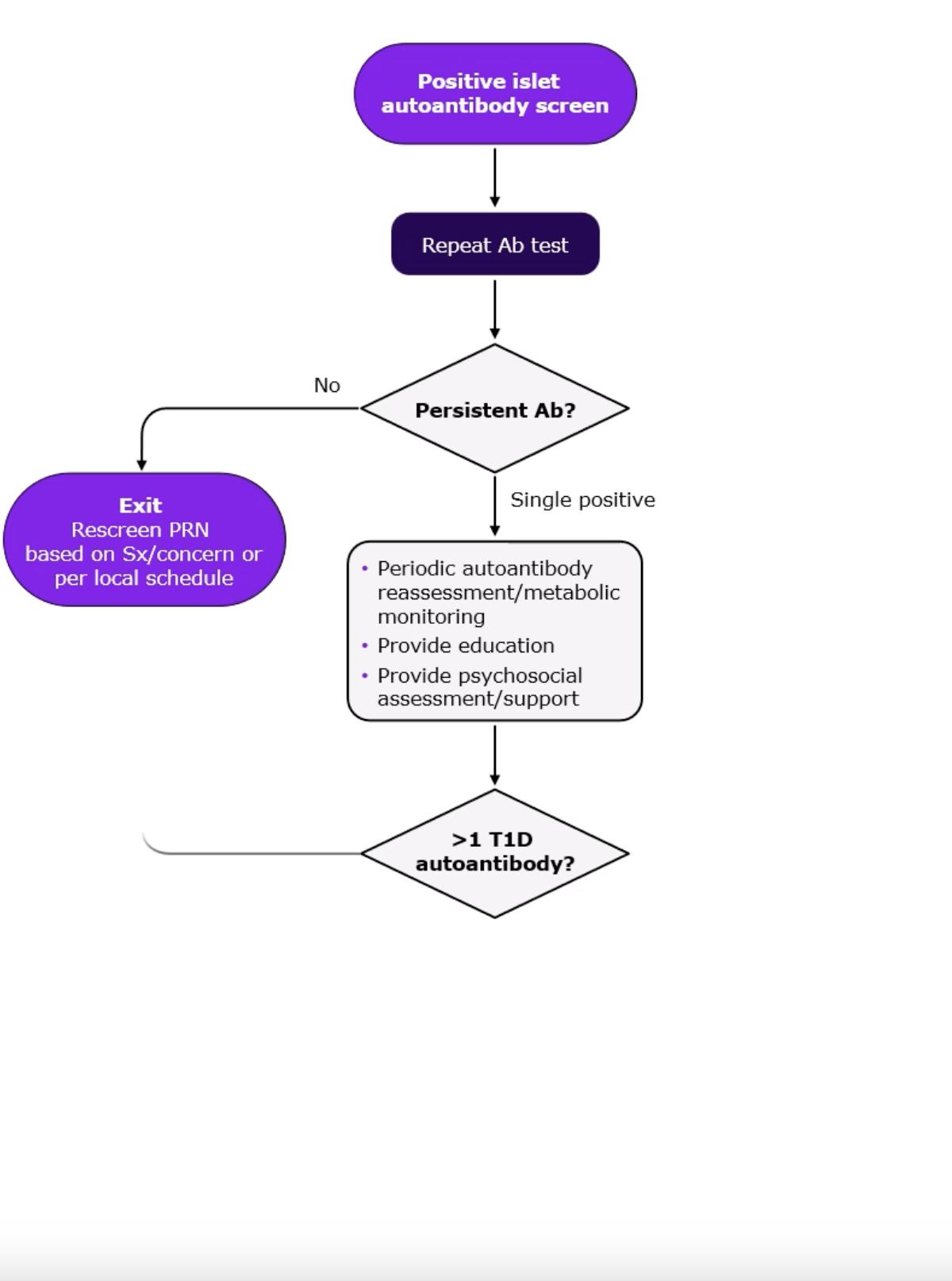
*Monitoring frequency and methodology depends on age, length of time since first detection of islet autoantibody, number of islet autoantibodies detected and presence of symptoms of type 1 diabetes
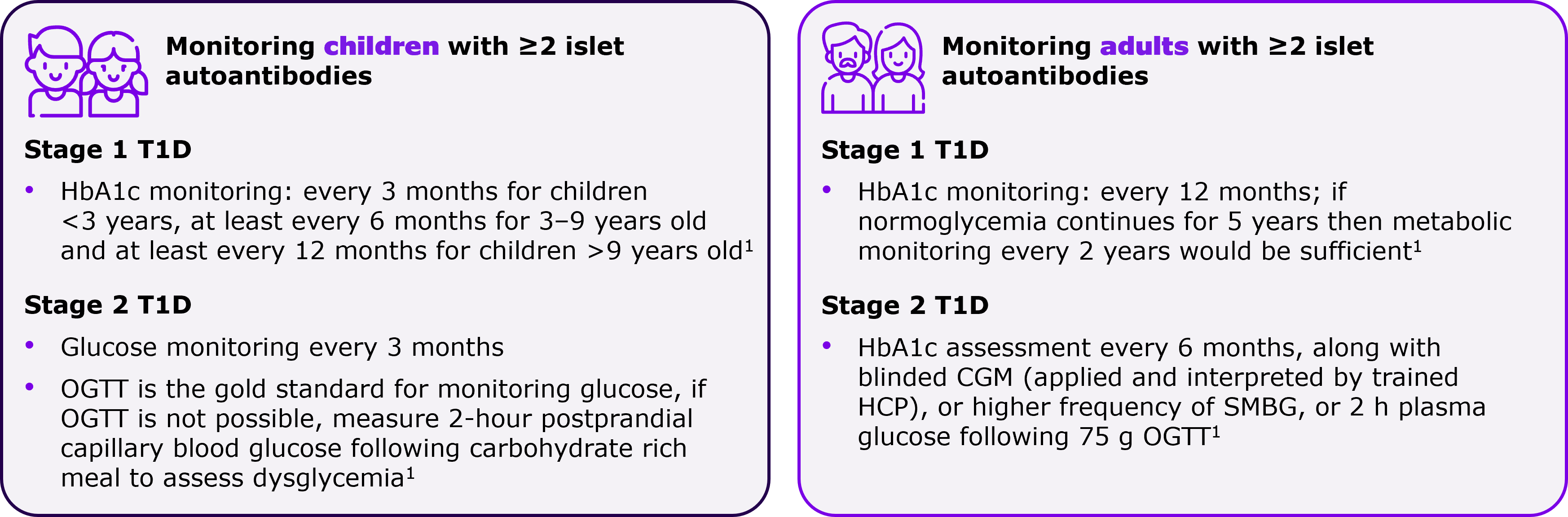
Are there any differences in metabolic monitoring approach in children versus adults?
Progression of autoimmune T1D among islet autoantibody-positive adults is slower than children, which is why there are minor variations in monitoring approaches.1 You can follow a more frequent metabolic monitoring for children compared to adults.1 Also, children who have progressed to Stage 2 autoimmune T1D need to be followed up in a specialty care setting.1 Refer to the figure below for more details.
Where should the monitoring be conducted?
Monitoring should be conducted wherever there are the necessary skills and resources to perform the required tests. Early stage (Stage 1 T1D) monitoring can be done in primary-care setting. As soon as you observe indicators of progression of disease to Stage 2 autoimmune T1D i.e. onset of glucose abnormalities, consider referring to specialty care provider. 1
Monitoring individuals who test positive for autoimmune T1D antibodies is crucial to reduce the risk of DKA and minimize the need for emergency care and hospitalization1
References
- Phillip M, Achenbach P, Addala A, et al. Consensus guidance for monitoring individuals with islet autoantibody-positive pre-stage 3 type 1 diabetes. Diabetologia. Published online June 24, 2024. doi:10.1007/s00125-024-06205-5
- Mameli C, Triolo TM, Chiarelli F, Rewers M, Zuccotti G, Simmons KM. Lessons and gaps in the prediction and prevention of type 1 diabetes. Pharmacol Res. 2023;193:106792. doi:10.1016/j.phrs.2023.106792
- Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38(10):1964-1974. doi:10.2337/dc15-1419
- Besser REJ, Bell KJ, Couper JJ, et al. ISPAD Clinical Practice Consensus Guidelines 2022: Stages of type 1 diabetes in children and adolescents. Pediatr Diabetes. 2022;23(8):1175-1187
- Greenbaum CJ. A Key to T1D Prevention: Screening and Monitoring Relatives as Part of Clinical Care. Diabetes. 2021;70(5):1029-1037. doi:10.2337/db20-1112
- Helminen O, Aspholm S, Pokka T, et al. OGTT and random plasma glucose in the prediction of type 1 diabetes and time to diagnosis. Diabetologia. 2015;58(8):1787-1796.
Do common genetic risk factors overlap for the autoimmune diseases?
Shared genetic risk factors and immune system dysregulation are potentially implicated both in polyautoimmunity and family autoimmunity among individuals with autoimmune T1D.2 Human leukocyte antigen (HLA) genotypes, PTPN22 gene, and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) — all contribute to the complex interplay of genetic factors in autoimmune diseases that overlap with autoimmune T1D, as depicted in the below figure.1
| Genotype |
Disease association | Additional information |
|
HLA DR3-DQ2 & |
|
|
|
MHC class I chain related gene A Polymorphism of MIC-A (especially alleles 5 and 5.1) |
|
|
|
Polymorphism of PTPN22 gene, encoding LYP |
| NA |
|
CTLA-4 |
|
|
21-OH; 21-hydroxylase; AITD autoimmune thyroid disease; CTLA-4, cytotoxic T lymphocyte-associated antigen-4; HLA, human leukocyte antigen; LYP, lymphoid tyrosine phosphatase; MIC-A, MHC-I related gene; T1D, type 1 diabetes.
ADA guideline recommendations for screening of autoimmune diseases in autoimmune T1D
Individuals with an existing autoimmune disease are more prone to develop other associated autoimmune diseases.4-6,10
The 2024 ADA guidelines on Comprehensive Medical Evaluation and Assessment of Comorbidities recommend screening for autoimmune thyroid disease soon after a T1D diagnosis and periodically, thereafter. Additionally, screening for celiac disease is recommended in the presence of gastrointestinal symptoms, signs, laboratory manifestations, or suspicion of celiac disease.10
To learn more about screening for autoantibodies to identify individuals at risk for autoimmune T1D, click here.
Screening for autoimmune T1D should be advised in individuals with other associated autoimmune diseases, given their higher risk than general population4‑6
References
- Barker JM. Clinical review: Type 1 diabetes-associated autoimmunity: natural history, genetic associations, and screening. J Clin Endocrinol Metab. 2006;91(4):1210–1217.
- Cardenas-Roldan J, Rojas-Villarraga A, Anaya JM. How do autoimmune diseases cluster in families? A systematic review and meta-analysis. BMC Med. 2013;11:73.
- Glowinska-Olszewska B, Szablowski M, Panas P, et al. Increasing co-occurrence of additional autoimmune disorders at diabetes type 1 onset among children and adolescents diagnosed in years 2010-2018-single-center study. Front Endocrinol (Lausanne). 2020;11:476.
- Ferrari SM, Fallahi P, Ruffilli I, Elia G, Ragusa F, Benvenga S, Antonelli A. The association of other autoimmune diseases in patients with Graves' disease (with or without ophthalmopathy): Review of the literature and report of a large series. Autoimmun Rev. 2019 Mar;18(3):287-292.
- Zelissen PM, Bast EJ, Croughs RJ. Associated autoimmunity in Addison's disease. J Autoimmun. 1995 Feb;8(1):121-30.
- Lauret E, Rodrigo L. Celiac disease and autoimmune-associated conditions. Biomed Res Int. 2013;2013:127589.
- Bao YK, Weide LG, Ganesan VC, et al. High prevalence of comorbid autoimmune diseases in adults with type 1 diabetes from the HealthFacts database. J Diabetes. 2019;11(4):273–279.
- Hemminki K, Li X, Sundquist J, Sundquist K. Familial association between type 1 diabetes and other autoimmune and related diseases. Diabetologia. 2009;52(9):1820-8.
- Besser REJ, Bell KJ, Couper JJ, et al. ISPAD clinical practice consensus guidelines 2022: Stages of type 1 diabetes in children and adolescents. Pediatr Diabetes. 2022;23:1175-87.
- American Diabetes Association Professional Practice C. 4. Comprehensive medical evaluation and assessment of comorbidities: Standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S52–S76.
MAT-GLB-2404800-1.0-08/2024