- Article
- Source: Campus Sanofi
- May 23, 2025
A video series on round table discussions featuring experts: Different inflammatory pathways in COPD impacting disease pathobiology and progression

COPD is a heterogenous and progressive disease, driven by distinct chronic immune processes. In a round table discussion, leading respiratory experts discussed
- What are the current unmet needs for patients with COPD?
- How do different inflammatory pathways in COPD impact disease pathobiology and progression?
- Biomarkers to guide COPD management.
The round table was chaired by Professor Paola Rogliani of the University of Rome, Italy. Other experts on the panel were:
Dr. MeiLan Han, Professor of Pulmonary Critical Care Medicine at the University of Michigan, Ann Arbor, MI, USA.
Dr. Marc Miravitlles, a chest physician from the Hospital Universitari Vall d’Hebron, Barcelona, Spain.
How do different inflammatory pathways in COPD impact disease pathobiology and progression?
Watch the video below to gain insights on this topic from the respiratory experts.
In this video, Dr. Han provides answers to a pertinent question from Prof. Rogliani, “How do different inflammatory endotypes of COPD impact disease pathogenesis and progression?”
COPD is a heterogenous disease
COPD is the end-result of complex, cumulative and dynamic gene-environment interactions over the lifetime that can damage the lungs and/or alter their normal developmental or aging processes.1
Inflammatory processes in COPD
Dr. Han remarked that traditionally, it is believed that COPD is driven by neutrophilic inflammation. COPD is typically associated with increased macrophages in peripheral airways, lung parenchyma and pulmonary vessels, along with activated neutrophils and lymphocytes.1 Neutrophils stimulate mucus hypersecretion and generate further oxidative stress, which can perpetuate inflammation.2
Airway damage following exposure to cigarette smoke and pollutants leads to an epithelial alarmins-mediated pro-inflammatory cascade, involving key cytokines such as IL-33, IL-25 and thymic stromal lymphopoietin (TSLP).3 Altered barrier function resulting from airway damage also predisposes the airway to infection, further amplifying the type 1 inflammatory cascade.3
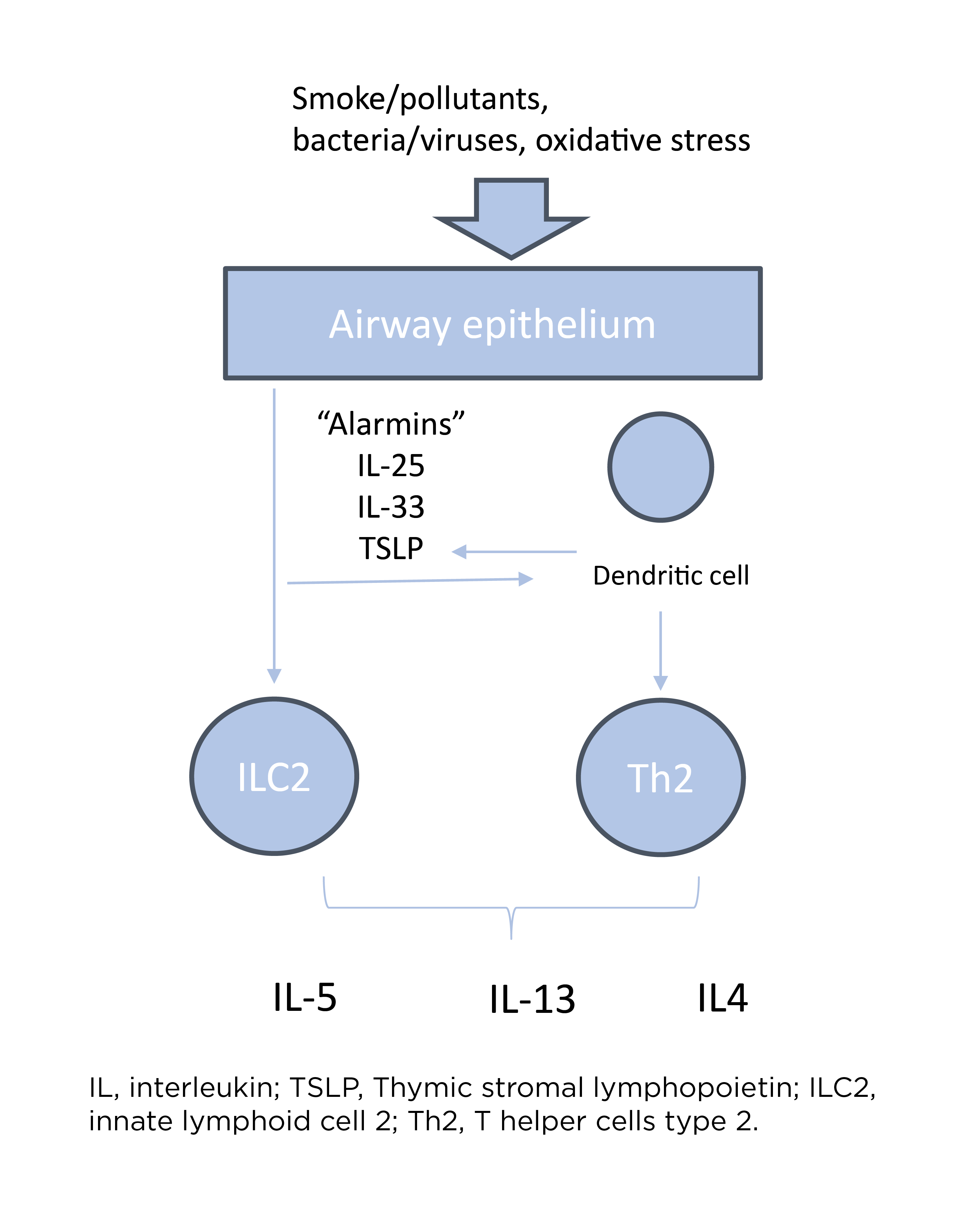
Type 2 pathway in COPD
Dr. Han highlighted, “In recent years, we've begun to better appreciate another inflammatory pathway that can be important in some patients with COPD, which is type 2 inflammation.”
Listen to an expert’s perspective on Decoding Type 2 Inflammation in COPD
Type 2 inflammation in COPD encompasses type 2 effector cells, including type 2 helper cells (Th2), innate lymphoid cells (ILC2), eosinophils and mast cells, as well as key cytokines such as IL-4, IL-5, and IL-13.4,5

Learn more about the Role of Type 2 Inflammation
COPD endotype with type 2 inflammation
A subset of patients with COPD and type 2 airway inflammation has been identified, even after excluding features of asthma.4,8 The prevalence of persistently elevated blood eosinophil count (BEC [EOS]) varies between studies, depending on population characteristics and threshold definitions used for evaluation.4
Read more about Blood Eosinophils as a Biomarker in Patients with COPD
In this context, Dr. Han discussed the ECLIPSE (Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points) cohort study, which demonstrated that 37% of patients with COPD (n=554/1483) had persistently elevated BEC (EOS) (≥2%).8
Referring to two studies in frequent exacerbators receiving triple therapy for COPD, Dr. Han pointed out that, “BEC (EOS) can be seen elevated in patients with frequent exacerbations.”
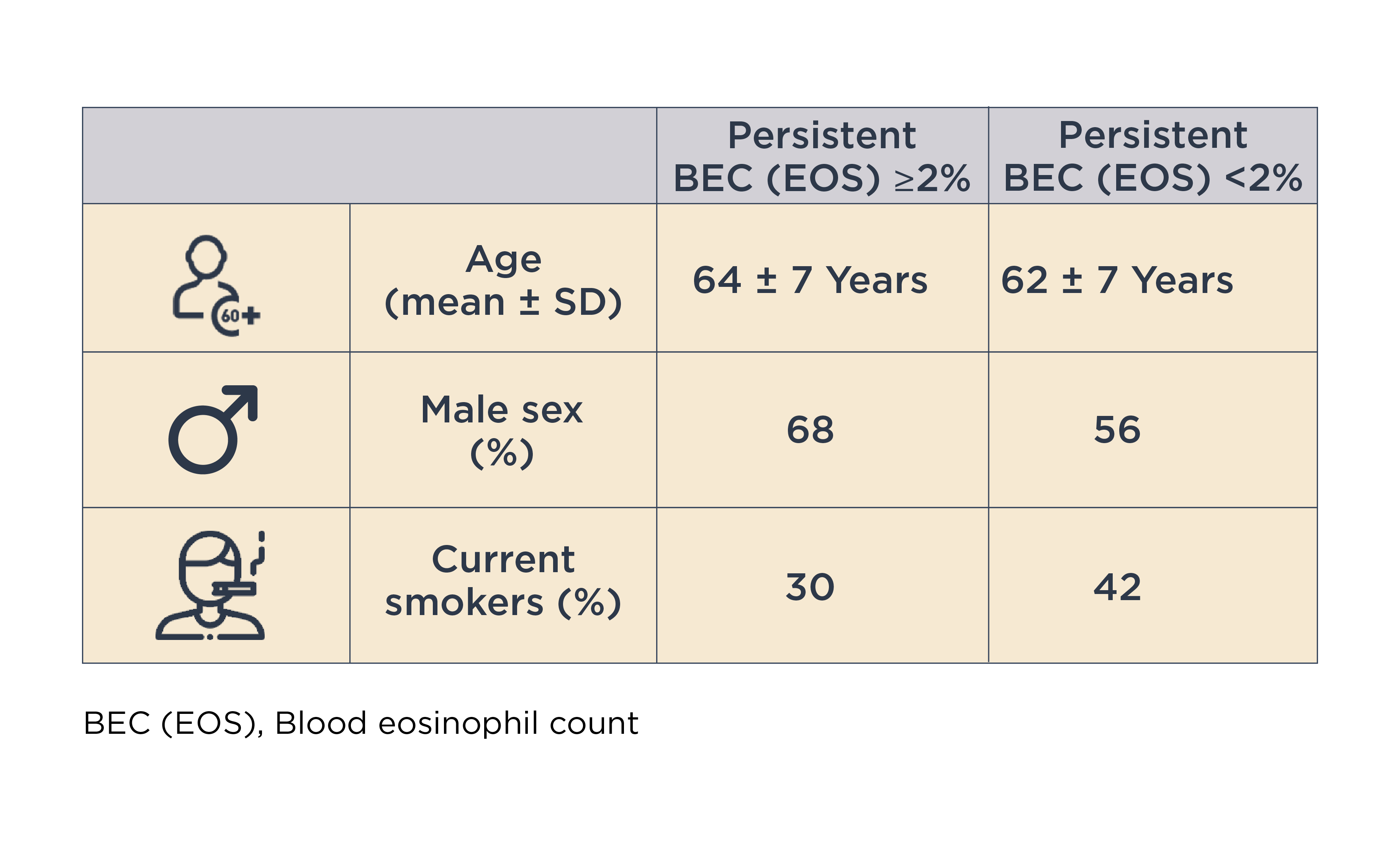
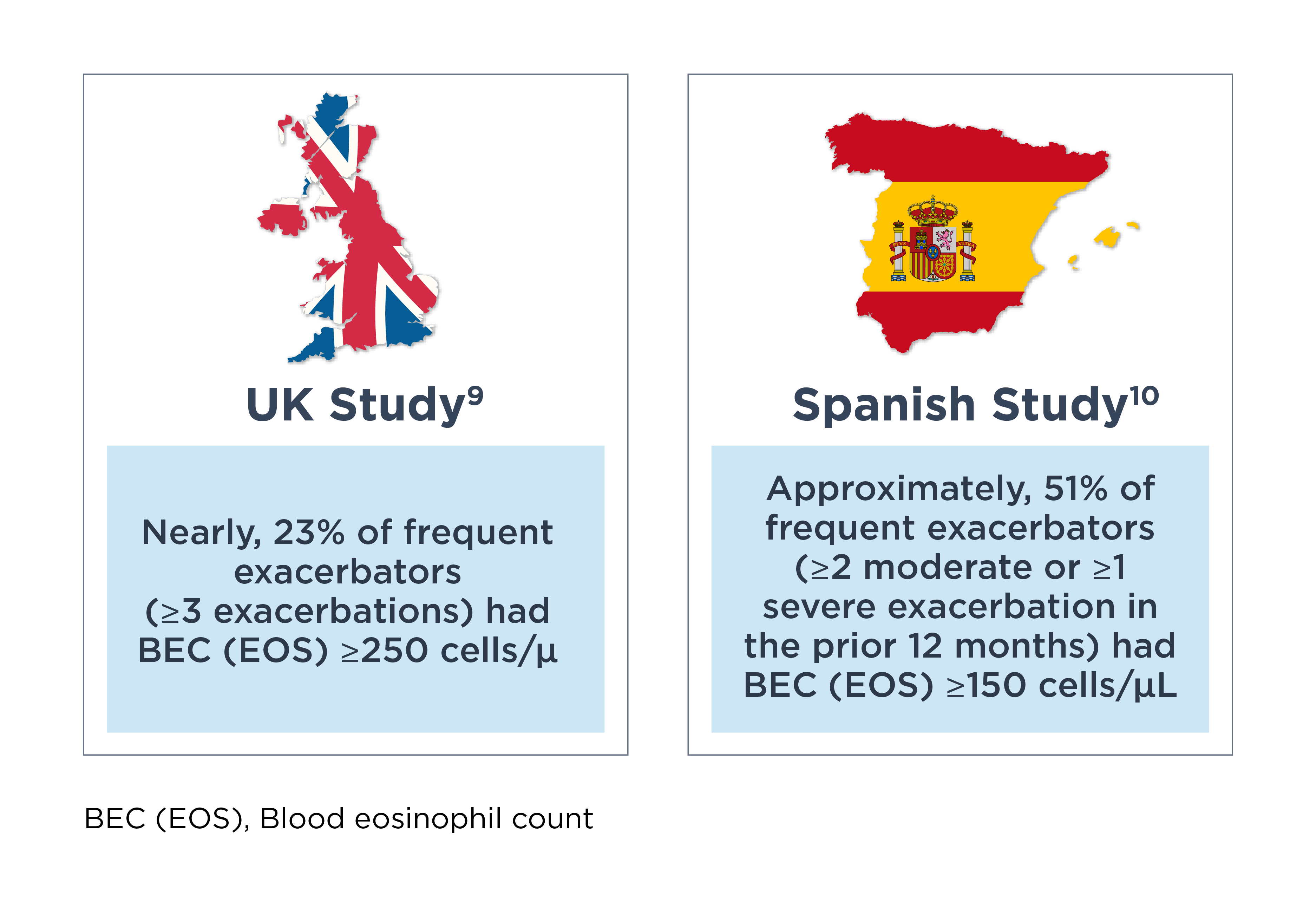
She also emphasized, “So this is actually an incredibly common finding in COPD.”
Panel discussion
Following Dr. Han’s presentation, the panel of experts put forth their opinions on the involvement of type 2 inflammation in COPD pathogenesis.
Dr. Rogliani pointed out that endotyping is a key to properly understanding the disease and how to eventually treat COPD patients.
She added that airway epithelium is the key aspect in determining the type of inflammatory profile a patient can exhibit because it will respond differently upon exposure to different stimuli such as pollution/smoking/infection, infection and smoking. “This will lead to a different cascade of inflammatory mediators, cytokines and also on cellular level such as eosinophils”, Dr. Rogliani further highlighted.
Dr. Miravitlles pointed out that the possibility of availability of biomarkers that can easily be used by all practicing physicians — not only specialists, but also in primary care — to identify patients with different endotypes, can help to move from bench to bedside (i.e. to move from the research arena to the clinical practice).
For more information, please read A review of the clinical implications of eosinophil stability and predictive capability in COPD
Dr. Rogliani agreed that it could be a good start to characterize the patient differently from the standpoint of inflammatory profile.
Dr. Han added that blood eosinophils are there for clinicians right now. She highlighted that patients really benefit clinically from steroids, but it is terrible that chronic use of steroids is associated with long term harms. Further, she added, “There is subset of patients which does have type 2 inflammation. We need to find better ways of identifying such patients because they really would benefit from targeted therapies.”
Dr. Rogliani concluded this topic by saying, “Identification of the right patient is the key in order to better treat and better approach COPD patients.”
References
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2025 report) (Accessed on May 13, 2025 at https://goldcopd.org/2025-gold-report/)
- Barnes PJ. Inflammatory endotypes in COPD. Allergy. 2019 Jul;74(7):1249-1256. doi: 10.1111/all.13760. Epub 2019 Mar 31. PMID: 30834543.
- Brightling C, Greening N. Airway inflammation in COPD: progress to precision medicine. Eur Respir J. 2019 Aug 1;54(2):1900651. doi: 10.1183/13993003.00651-2019. PMID: 31073084.
- Oishi K, Matsunaga K, Shirai T, Hirai K, Gon Y. Role of Type2 Inflammatory Biomarkers in Chronic Obstructive Pulmonary Disease. J Clin Med. 2020 Aug 18;9(8):2670. doi: 10.3390/jcm9082670. PMID: 32824775; PMCID: PMC7464674.
- Maspero J, Adir Y, Al-Ahmad M, Celis-Preciado CA, Colodenco FD, Giavina-Bianchi P et al. Type 2 inflammation in asthma and other airway diseases. ERJ Open Res. 2022 Aug 1;8(3):00576-2021. doi: 10.1183/23120541.00576-2021. PMID: 35923421; PMCID: PMC9339769.
- Gandhi NA, Bennett BL, Graham NM, Pirozzi G, Stahl N, Yancopoulos GD. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016 Jan;15(1):35-50. doi: 10.1038/nrd4624. Epub 2015 Oct 16. PMID: 26471366.
- Doyle AD, Mukherjee M, LeSuer WE, Bittner TB, Pasha SM, Frere JJ, et al. Eosinophil-derived IL-13 promotes emphysema. Eur Respir J. 2019 May 30;53(5):1801291. doi: 10.1183/13993003.01291-2018. PMID: 30728205; PMCID: PMC7313423.
- Singh D, Kolsum U, Brightling CE, Locantore N, Agusti A, Tal-Singer R; ECLIPSE investigators. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014 Dec;44(6):1697-700. doi: 10.1183/09031936.00162414. Epub 2014 Oct 16. PMID: 25323230.
- David B, Bafadhel M, Koenderman L, De Soyza A. Eosinophilic inflammation in COPD: from an inflammatory marker to a treatable trait. Thorax. 2021 Feb;76(2):188-195. doi: 10.1136/thoraxjnl-2020-215167. Epub 2020 Oct 29. PMID: 33122447; PMCID: PMC7815887.
- Chen S, Miravitlles M, Rhee CK, Pavord ID, Jones R, Carter V, et al. Patients with Chronic Obstructive Pulmonary Disease and Evidence of Eosinophilic Inflammation Experience Exacerbations Despite Receiving Maximal Inhaled Maintenance Therapy. Int J Chron Obstruct Pulmon Dis. 2022 Sep 9;17:2187-2200. doi: 10.2147/COPD.S378649. PMID: 36110306; PMCID: PMC9470244.
- Alcázar-Navarrete B, García-Rio F, Sánchez G, Mariscal E, García A, Cuesta M, et al. Burden of Disease Among Exacerbating Patients with COPD Treated with Triple Therapy in Spain. Int J Chron Obstruct Pulmon Dis. 2021 Jul 21;16:2149-2161. doi: 10.2147/COPD.S310319. PMID: 34321874; PMCID: PMC8312318.
MAT-GLB-2400917