ALPROLIX® prophylaxis helps patients achieve bleed protection* with confidence1,2
*ALPROLIX has been proven to help patients prevent bleeding episodes using a prophylaxis regimen.1
PTPs ≥12 years in the B-LONG trial



42% of adult and adolescent PTPs had an ABR of 0 on ALPROLIX in B-LONG3†‡
†Interval-adjusted prophylaxis arm for B-LONG.1
‡11 out of the 26 evaluated patients in the IP arm of B-LONG had an ABR of 0.3
ABR=annualized bleed rate; AsBR=annualized spontaneous bleed rate; IP=individualized prophylaxis; PTP=previously treated patient.
CLINICAL TRIAL DESIGN: ALPROLIX WAS DEMONSTRATED EFFECTIVE IN THE B-LONG TRIAL1
B-LONG was a phase 3 open-label study investigating the safety and efficacy of ALPROLIX in 123 adult and adolescent PTPs with severe hemophilia B. Study arms included: fixed-interval (weekly) (n=63), fixed-dose (interval-adjusted) (n=29), on-demand (n=27), and surgical (n=12).1
A longitudinal analysis revealed ABRs remained low for patients taking ALPROLIX prophylaxis4

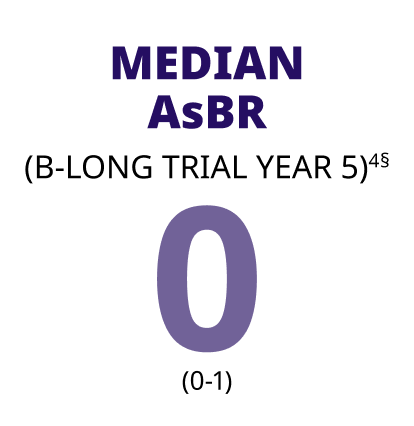
.png)
- A target joint was defined as a major joint with ≥3 bleeding episodes over 3 consecutive months4
- Target joint resolution was defined as ≤2 spontaneous joint bleeds within 12 months4
§In a 5-year subanalysis of the interval-adjusted prophylaxis arm of B-YOND, 17 patients ≥12 years achieved a median ABR of 1 (0-2.0) for Year 5, and a median AsBR of 0 (0-1.0) for Year 5.4
ABR=annualized bleed rate; AsBR=annualized spontaneous bleed rate; PTP=previously treated patient.
ALPROLIX was studied for up to 6.5 years in adult and adolescent patients, from the start of the B-LONG trial to the end of the B-YOND trial4
CLINICAL TRIAL DESIGN: ALPROLIX HAS PROVEN EFFICACY ACROSS TRIALS1,5
Kids B-LONG was a phase 3 open-label study investigating the safety and efficacy of ALPROLIX in 30 PTPs aged ≤11 years with severe hemophilia. The number of patients 1 to 5 years of age was 15, and 6 to 11 years of age was 15. All 30 patients were treated with ALPROLIX on an individualized prophylactic regimen.1
B-YOND was an open-label extension trial that studied the long-term safety and efficacy of ALPROLIX over 5 years in 120 adult, adolescent, and pediatric patients previously treated in Kids B-LONG or B-LONG. Study arms included: fixed-interval (n=74), fixed-dose (n=36), modified prophylaxis (n=17), and on-demand (n=15).5
ALPROLIX offers acute bleed control your on-demand patients can trust, no matter their age1,6
ALPROLIX resolved most bleeds with 1 or 2 infusions1,6
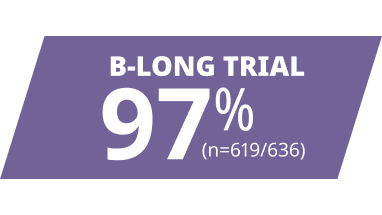
Response to first infusion was rated good or excellent1,6||
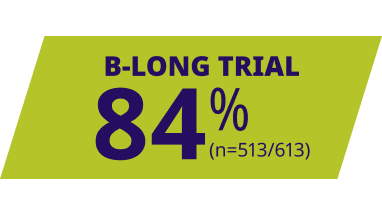
||Excellent response was defined as abrupt pain relief and/or improvement in signs of bleeding. Good response was defined as definite pain relief and/or improvement in signs of bleeding but possibly requiring another injection in 1 or 2 days.1
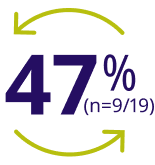
47% of patients ≥12 years treated with ALPROLIX on-demand during the B-LONG trial switched to ALPROLIX prophylaxis during the B-YOND trial5
Perioperative dosing offers hemostatic control for major and minor surgeries1
Spotlight on Surgical Patients ≥12 years1,6
While the ALPROLIX surgical data were pooled, here is how the adult and adolescent subpopulation is reflected in these numbers:
- Major surgeries in adult & adolescent patients: 14 (B-LONG Trial) + 20 (B-YOND Trial)
- Minor surgeries in adult & adolescent patients: 15 (B-LONG Trial) + 42 (B-YOND Trial)
Perioperative efficacy across all ages1,6

|
35 MAJOR SURGERIES6 |
62 MINOR SURGERIES6 | ||
|
Joint replacement/revision (n=10) |
Tooth extraction (n=24) | ||
|
Abdominal (n=6) |
Eye surgery (n=5) | ||
|
Other orthopedic (n=5) |
Oral surgery (n=5) | ||
|
Fracture and fixation (n=3) |
Incision and drainage (n=5) | ||
|
Arthroscopy (n=2) |
Vascular procedure (n=5) | ||
|
Spinal surgery (n=2) |
Minor orthopedic (n=4) | ||
|
Cranial/brain (n=2) |
Other non-orthopedic (n=4) | ||
|
Other non-orthopedic (n=2) |
Other dental (n=4) | ||
|
Joint fusion (n=2) |
Port placement or removal (n=3) | ||
|
Dental (n=1) |
Minor skin procedure (n=2) | ||
|
|
Endoscopy with/without procedure (n=1) | ||
|
22 patients1,6|| |
37 patients1,6|| |
||Patients were classified by parent study. Data were derived from surgeries performed during the B-LONG trial, Kids B-LONG trial, and B-YOND extension trial. Those who underwent major and minor surgery were included in both cohorts. Eight subjects had more than one major surgery.1,6
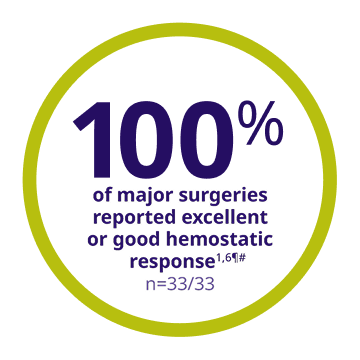
Patients undergoing major surgery had an excellent or good hemostatic response with ALPROLIX1
Perioperative efficacy across all ages
- The median average dose per injection to maintain hemostasis during surgery was 94.7 IU/kg (range: 49 to 152). Perioperative factor IX replacement with ALPROLIX was by bolus infusion only. The safety of continuous infusion was not evaluated1
- 80% (28 of 35) of the major surgeries required a single perioperative dose to maintain hemostasis during surgery1
- Hemostasis was assessed by the investigator after surgery1
MAJOR SURGERIES:
B-LONG, KIDS B-LONG, AND B-YOND TRIALS
MINOR SURGERIES:
B-LONG, KIDS B-LONG, AND B-YOND TRIALS
¶Out of 35 major surgeries, 33 were assessed in 22 subjects.1,6
#8 subjects had more than 1 major surgery.1,6
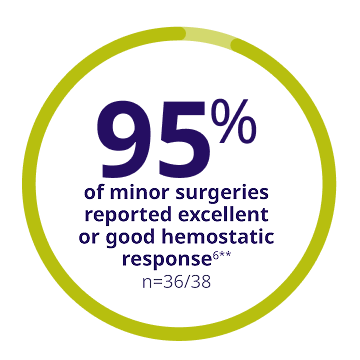
**Out of 62 minor surgeries, 38 were assessed in 37 subjects.6
ALPROLIX offers acute bleed control your on-demand patients can trust, no matter their age1,6
ALPROLIX resolved most bleeds with 1 or 2 infusions1,6

Response to first infusion was rated good or excellent1,6||

||Excellent response was defined as abrupt pain relief and/or improvement in signs of bleeding. Good response was defined as definite pain relief and/or improvement in signs of bleeding but possibly requiring another injection in 1 or 2 days.1
47% of patients ≥12 years treated with ALPROLIX on-demand during the B-LONG trial switched to ALPROLIX prophylaxis during the B-YOND trial5

Perioperative dosing offers hemostatic control for major and minor surgeries1
Spotlight on Surgical Patients ≥12 years1,6
While the ALPROLIX surgical data were pooled, here is how the adult and adolescent subpopulation is reflected in these numbers:
- Major surgeries in adult & adolescent patients: 14 (B-LONG Trial) + 20 (B-YOND Trial)
- Minor surgeries in adult & adolescent patients: 15 (B-LONG Trial) + 42 (B-YOND Trial)
Perioperative efficacy across all ages1,6

|
35 MAJOR SURGERIES6 |
62 MINOR SURGERIES6 | ||
|
Joint replacement/revision (n=10) |
Tooth extraction (n=24) | ||
|
Abdominal (n=6) |
Eye surgery (n=5) | ||
|
Other orthopedic (n=5) |
Oral surgery (n=5) | ||
|
Fracture and fixation (n=3) |
Incision and drainage (n=5) | ||
|
Arthroscopy (n=2) |
Vascular procedure (n=5) | ||
|
Spinal surgery (n=2) |
Minor orthopedic (n=4) | ||
|
Cranial/brain (n=2) |
Other non-orthopedic (n=4) | ||
|
Other non-orthopedic (n=2) |
Other dental (n=4) | ||
|
Joint fusion (n=2) |
Port placement or removal (n=3) | ||
|
Dental (n=1) |
Minor skin procedure (n=2) | ||
|
|
Endoscopy with/without procedure (n=1) | ||
|
22 patients1,6|| |
37 patients1,6|| |
||Patients were classified by parent study. Data were derived from surgeries performed during the B-LONG trial, Kids B-LONG trial, and B-YOND extension trial. Those who underwent major and minor surgery were included in both cohorts. Eight subjects had more than one major surgery.1,6
Patients undergoing major surgery had an excellent or good hemostatic response with ALPROLIX1
Perioperative efficacy across all ages
- The median average dose per injection to maintain hemostasis during surgery was 94.7 IU/kg (range: 49 to 152). Perioperative factor IX replacement with ALPROLIX was by bolus infusion only. The safety of continuous infusion was not evaluated1
- 80% (28 of 35) of the major surgeries required a single perioperative dose to maintain hemostasis during surgery1
- Hemostasis was assessed by the investigator after surgery1
MAJOR SURGERIES:
B-LONG, KIDS B-LONG, AND B-YOND TRIALS

MINOR SURGERIES:
B-LONG, KIDS B-LONG, AND B-YOND TRIALS

¶Out of 35 major surgeries, 33 were assessed in 22 subjects.1,6
#8 subjects had more than 1 major surgery.1,6
**Out of 62 minor surgeries, 38 were assessed in 37 subjects.6
*ALPROLIX has been proven to help patients prevent bleeding episodes using a prophylaxis regimen.1
INDICATION:
References: 1. ALPROLIX. Package insert. Bioverativ Therapeutics Inc; 2023. 2. Powell JS, Pasi KJ, Ragni MV, et al; B-LONG Investigators. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N Engl J Med. 2013;369(24):2313-2323. 3. Data on file. Waltham, MA; Bioverativ Therapeutics Inc. 4. Shapiro AD, Kulkarni RD, Ragni MV, et al. Post hoc longitudinal assessment of efficacy and safety of recombinant factor IX Fc fusion protein in hemophilia B. Blood Adv. 2023;7(13):3049-3057. 5. Pasi KJ, Fischer K, Ragni M, et al. Long-term safety and sustained efficacy for up to 5 years of treatment with recombinant factor IX Fc fusion protein in subjects with haemophilia B: results from the B-YOND extension study. Haemophilia. 2020;26(6):e262-e271. 6. Chowdary P, Holmström M, Mahlangu JN, et al. Managing surgery in hemophilia with recombinant factor VIII Fc and factor IX Fc: Data on safety and effectiveness from phase 3 pivotal studies. Res Pract Thromb Haemost. 2022;6(5):e12760.

