- Article
- Source: Campus Sanofi
- May 20, 2025
Nirsevimab : Clinical Experience
_430x268.jpg)
Study Design
Preclinical trials
2014
Phase 1a1
NCT02114268
“1st Time in Healthy Adults”
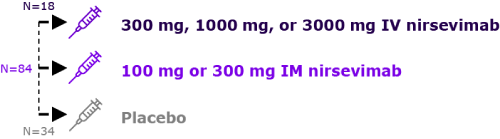.png)

Healthy adults aged 18 to 49 years (n=136)

Evaluation of pharmacokinetics and safety profile of Nirsevimab before initiating a clinical study in infants
Phase 1b/2a2
NCT02290340
“1stTime in Healthy Preterm Infants”
.png)

Healthy preterm infants 32-35 wGA (n=89)

Evaluation of pharmacokinetics and safety profile of Nirsevimab in healthy preterm infants
Pivotal clinical trials
2016
Phase 2b3
NCT02878330
“Infants not eligible to receive Palivizumab as per AAP / other guidelines”


Healthy preterm infants in healthy infants 29-34 weeks 6 days wGA (n=1453)

Evaluation of nirsevimab for the prevention of RSV-associated lower respiratory tract infection in healthy infants
2019
Phase 34,5
NCT03979313
mobile.png)


Healthy late preterm and term infants ≥ 35 wGA (n=3012)

Evaluation of efficacy and safety of nirsevimab in healthy late-preterm and term infants entering their first RSV season
Due to COVID-19, no RSV cases were observed. Therefore, a joint decision with health authorities was taken to analyze the primary endpoint (primary cohort). MELODY trial restarted to further characterize nirsevimab safety in this population (secondary cohort)
2019
Phase 2/36-8
NCT03959488
‘MEDLEY’
Preterm cohort
(n=615)
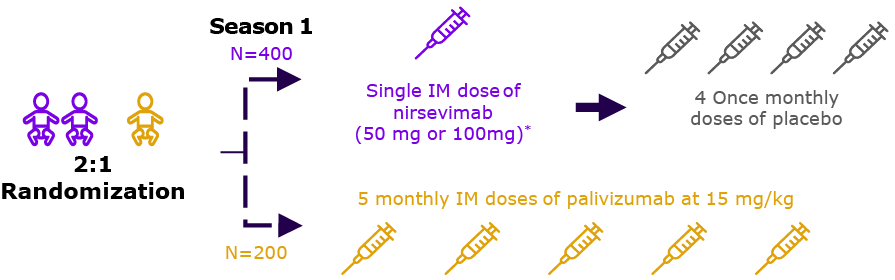

Evaluation of safety of nirsevimab in preterm infants with OR without CHD or CLD of prematurity
2019
Season 2† (n=262)
CHD/CLD cohort
(n=310)
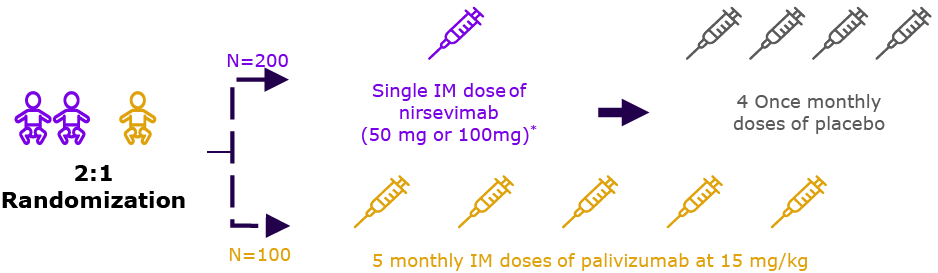
2020
Phase 29,31
NCT04484935
‘MUSIC’
.png)
.png)
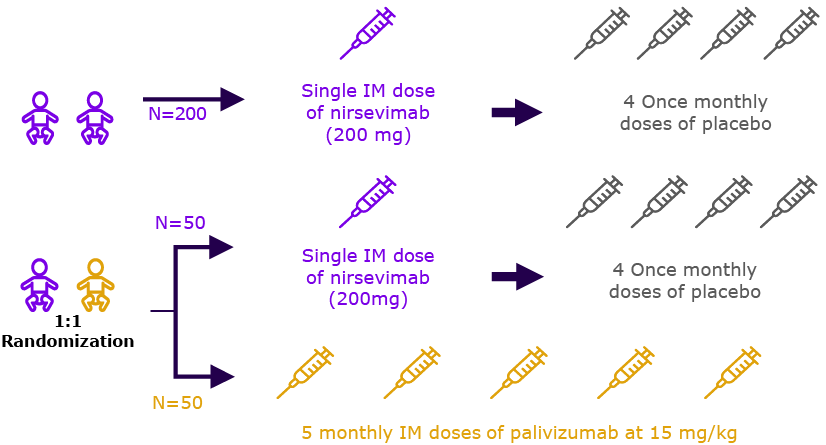
Immunocompromised children who are ≤ 24 months of age at the time of dose administration. (n=100)

Evaluation of safety and tolerability, for nirsevimab in immunocompromised children
2022
Phase 3b10
NCT05437510
‘HARMONIE’
Healthy infants ≥29 wGA not eligible for palivizumab (n=8058)


Determination of efficacy and safety of nirsevimab for the prevention of hospitalizations due to RSV-LRTI in all palivizumab ineligible infants under 12 months
Key results from the clinical development program of nirsevimabs
Safety
Nirsevimab (N=3580): Favorable Safety Profile Across All Infants in pivotal studies
| Variables | Serious adverse events | Adverse events of Grade 3 or higher | Adverse events of special interest (AESI) | Deaths | ||
| Ph2b3 29-<35 wGA | Placebo (N=479) | 16.9% | 12.5% | 0.6% | 3 | |
| Nirsevimab (N=968) | 11.2% | 8.0% | 0.5% | 2 | ||
| MELODY4 ≥35 wGA | Placebo (N=996) | 7.4% | 3.8% | 0.0% | 0 | |
| Nirsevimab (N=1998) | 6.3% | 3.1% | 0.2% | 4 | ||
| MEDLEY First season6 | Preterm | Palivizumab (N=206) | 5.3% | 3.4 | 0.0% | 0 |
| Nirsevimab (N=406) | 6.9% | 3.4% | 0.2% | 2 | ||
| CHD/CLD | Palivizumab (N=98) | 20.4% | 13.3% | 0.0% | 1 | |
| Nirsevimab (N=208) | 19.2% | 14.4% | 0.5% | 3 | ||
- None of the serious adverse events or deaths were considered as related to nirsevimab
- Overall, incidence of nirsevimab antidrug antibody was low across studies with no safety concerns
- MELODY: Four AESI cases of hypersensitivity limited to cutaneous signs and symptoms
- MEDLEY: 2 AESIs (nirsevimab arm): Maculopapular rash (preterm cohort) 92 days post nirsevimab dose and heparin-induced thrombocytopenia (CHD/CLD cohort) unrelated to treatment
Clinical experience of nirsevimab continues with HARMONIE, MUSIC, and MELODY
| HARMONIE10,11 | MUSIC9 | MEDLEY Second season12 | ||||
| CHD/CLD | ||||||
| Variables | No intervention (N=4020) | Nirsevimab (N=4016) | Nirsevimab (N=100) | P/P (N=42) | P/N (N=40) | N/N (N=180) |
| Serious adverse events | 1.7% | 2.2% | 30% | 0% | 10% | 9.4% |
| Adverse events of Grade 3 or higher | 1.1% | 1.2% | 31.7 | 31.7 | 10% | 7.8% |
| Adverse events of special interest (AESI) | <0.1 | <0.1 | 6.7% | 6.7% | 0.0% | 0% |
| Deaths | 0 | 0 | 1 | 1 | 0 | 0 |
- Overall incidence of adverse events (AEs)13,14
- Serious AEs and treatment-related AEs were balanced between Nirsevimab and placebo groups
- No anaphylaxis or other serious allergic reactions
- No thrombocytopenia attributed to study drug
- No immune complex disease
- Nonserious cutaneous hypersensibility reactions occurred in 0.2% of nirsevimab recipients
- Levels of ADA were low
- Incidence of deaths were low and similar between groups
- None were considered treatment-related
Key results from the clinical development program
Phase
1a1
Nirsevimab administration resulted in a 4X increase in neutralizing antibodies persisting until day 181
(ranging from 50% in 100 mg IM cohort to 83% in 3000 mg IV cohort)
Phase
1b/2a2
The extended half-life and the demonstrated RSV-neutralizing activity supported the potential for protection against RSV
disease for the duration of a typical 5-month season with a single 50 mg IM dose of nirsevimab
Consistent efficacy against RSV-LRTI and associated hospitalizations
| RSV MA-LRTI | RSV LRTI Hospitalization | RSV Very severe MA-LRTI | All Cause LRTI Hospitalization | All Cause MA-LRTI | |
| Phase 2b3,15 | 70.1% (95% CI, 52.3-81.2) | 78.4% (95% CI, 51.9-90.3) | 87.5% (95% CI, 62.9, 95.8) | 42.5% (95% CI, 16.3-60.5) | 23.5% (95% CI, 7.1-37.0) |
| MELODY STUDY All | 76.4% (95% CI, 62.3–85.2) | 76.8% (95% CI, 49.4–89.4) | 78.6% (95% CI, 48.8–91.0) | 38.9% (95% CI, 6.3-60.2) | 38.2% (95% CI, 23.7-50.0) |
|
Phase 2b | 79.0% (95% CI, 68.5-86.1) | 80.6% (95% CI, 62.3-90.1) | 86.2% (95% CI, 68.1-94.0) | ||
| HARMONIE STUDY10 | 83.2% (95% CI, 67.7-92.0) | 75.7% (95% CI, 32.8-92.9) | 58.0% (95% CI, 39.7-71.2) |
All infants need protection from RSV16-21.

Nirsevimab is designed to provide protection for all infants for the length of typical RSV season with a single dose3,22.
Nirsevimab has demonstrated an efficacy of 79% against RSV-MA-LRTI (MELODY/Ph2b pooled), and 83% against hospitalizations (HARMONIE), for 150 days5,10.

Nirsevimab is the first-in-class and only prevention strategy approved by FDA and EMA and is designed to protect all infants from RSV-LRTI in their first RSV season23-25.
Regulatory approvals

NITAGs Recommend Nirsevimab for All Infants
.png)
Advisory Committee on Immunization Practices28
- First RSV Season: All infants below 8 months of age
- Second RSV Season: Infants and children (8-19 months) at increased risk of severe RSV
%20(1)%20(1).png)
Haute Autorité de santé29
- First RSV Season: All infants with reimbursements
%20(1)%20(1).png)
inisterio de Sanidad30
- First RSV Season: All infants below 6 months of age
- Second RSV Season: High risk under 24 months
AAP: American Academy of Paediatrics; AESI: adverse event of special interest; CHD: Chronic Heart Disease; CLD: Chronic Ling Disease; FDA: Food and Drug Administration; EMA: European Medicines Agency; IM: Intramuscular; IV: Intravenous; LRTI: lower respiratory tract infection; RSV: respiratory syncytial virus; wGA: weeks of gestational age.
-
Griffin MP, et al. Antimicrob Agents Chemother. 2017;61(3):e01714-16.
-
Domachowske JB, et al. Pediatr Infect Dis J. 2018;37(9):886-892.
-
Griffin MP, et al; N Engl J Med. 2020;383(5):415-425.
-
Muller WJ, et al. N Engl J Med. 2023;388(16):1533-1534.
-
Nirsevimab for the prevention of RSV in all infants Accessed October 2023.
-
Domachowske J, et al. N Engl J Med. 2022;386(9):892-894.
-
NCT03959488. Accessed October 2023.
-
Domachowske JB. et al. ReSViNET’s 7th Conference (RSVVW 2023), 22- 24 February 2023.
-
Mori et al. Asian Congress of Pediatric Infectious Diseases, 26-28 October 2022, Seoul, Korea.
-
SB Drysdale, A Phase 3 randomized open-label study of nirsevimab (versus no intervention) in preventing hospitalizations due to respiratory syncytial virus (RSV) in infants (HARMONIE). ESPID 2023: Lisbon, Portugal.
-
SN Faust, (2023, Oct 10-15th). The Impact of Nirsevimab on an RSV Season in All Infants: Data From The HARMONIE Study [Oral presentation]. ID Week 2023 Boston, MA, USA
-
Domachowske JB, et al. J Pediatric Infect Dis Soc. 2023;12(8):477-480.
-
Beyfortus. Summary of Product Characteristics. November 2022.
-
Mankad V. Comprehensive summary of all safety data of nirsevimab in healthy infants: experience to date from pivotal trials. ESPID 2023 (May 8–12, 2023); Lisbon, Portugal.
-
Muller WJ. Safety and efficacy of nirsevimab for prevention of medically attended RSV lower respiratory tract infection in all infants enrolled in the phase 3 melody trial [Oral presentation]. RSVVW 2023: Lisbon, Portugal. (February 22–24, 2023) : Lisbon, Portugal.
-
Leader S, et al. Pediatr Infect Dis J. 2002;21(7):629-632.
-
Karron R. Plotkin’s Vaccines. 7th ed. Philadelphia, PA : Elsevier;2018:943- 949.
-
Glezen WP, et al. Am J Dis Child. 1986;140(6):543-546.
-
Hall CB, et al. N Engl J Med. 2009;360(6):588-598.
-
Hall CB, et al. Pediatrics. 2013;132(2):e341-e348.
-
Rha B, et al. Pediatrics. 2020;146(1):e20193611.
-
Hammitt LL, et al. N Engl J Med. 2022;386(9):837-846.
-
Sanofi presentation to US Advisory Committee on Immunization Practices. 20 October 2022. Accessed October 2023.
-
FDA approves Beyfortus™(nirsevimab-alip) to protect infants against RSV disease. Accessed October 2023.
-
European Medicines Agency. Nirsevimab. EMEA/H/C/005304. Accessed October 2023.
-
Medicines & Healthcare products Regulatory Agency (MHRA). MHRA- 100067-PIP01-21-M01. Accessed October 2023.
-
Health Canada approves BEYFORTUS™ (nirsevimab) for the prevention of RSV disease in infants Accessed October 2023.
-
Jones JM, et al. MMWR Morb Mortal Wkly Rep. 2023;72(34):920-925.
-
HAS | BEYFORTUS (nirsevimab) - Respiratory syncytial virus Accessed October 2023.
-
Recomendaciones de utilización de nirsevimab frente a virus respiratorio sincitial para la temporada 2023-2024 Accessed October 2023.
-
History of Changes for Study - NCT04484935 Accessed October 2023.
MAT-SA-2400215-V1-April 2024