- Article
- Source: Campus Sanofi
- Sep 30, 2025
There's a Beta Way to look at type 1 diabetes

Because pancreatic beta cell reserve determines the course of the T1D journey1-7
Autoimmune type 1 diabetes (T1D) is driven by the progressive and irreversible loss of beta cells.8-11
While exogenous insulin therapy is effective, it cannot fully replicate the full spectrum of functions beta cells perform in maintaining glucose homeostasis.12-14
The profound implications of this are clearly demonstrated:
- Patients with more vs less residual beta cell function, as indicated by C-peptide, are at lower risk of severe hypoglycaemia, diabetic ketoacidosis (DKA) and long-term complications.1-6,15-17,19
- While more betas are better, even limited residual activity can still positively impact the patient experience of T1D.1-7
As such, it is the pancreatic beta cell reserve that determines the course of the autoimmune T1D journey.1-7
It's time to recognise the role of beta cells beyond insulin. It’s time to recognise the value of every single beta cell.
Mechanisms of disease in autoimmune T1D
The progressive, irreversible decline of functional beta cell mass in autoimmune T1D can be detected up to 6 years before symptoms arise.8-11,19,20 Autoantibody screening in this critical window of opportunity can help identify individuals at risk of progression to Stage 3 T1D, enabling a smoother transition to life with the condition.21-27
T1D is an autoimmune condition characterised by the progressive and irreversible loss of beta cells8-11
This loss drives8,10,28:
- metabolic failure
- vascular complications
- and life-long dependence on replacement insulin therapy
The condition manifests in four distinct stages as functional beta cell mass progressively declines.23,29,30 Stages are defined by the presence of autoantibodies, level of glycaemic control, symptoms, insulin dependence and duration of diabetes.21,23,30
The four stages of autoimmune T1D21,23,29,30
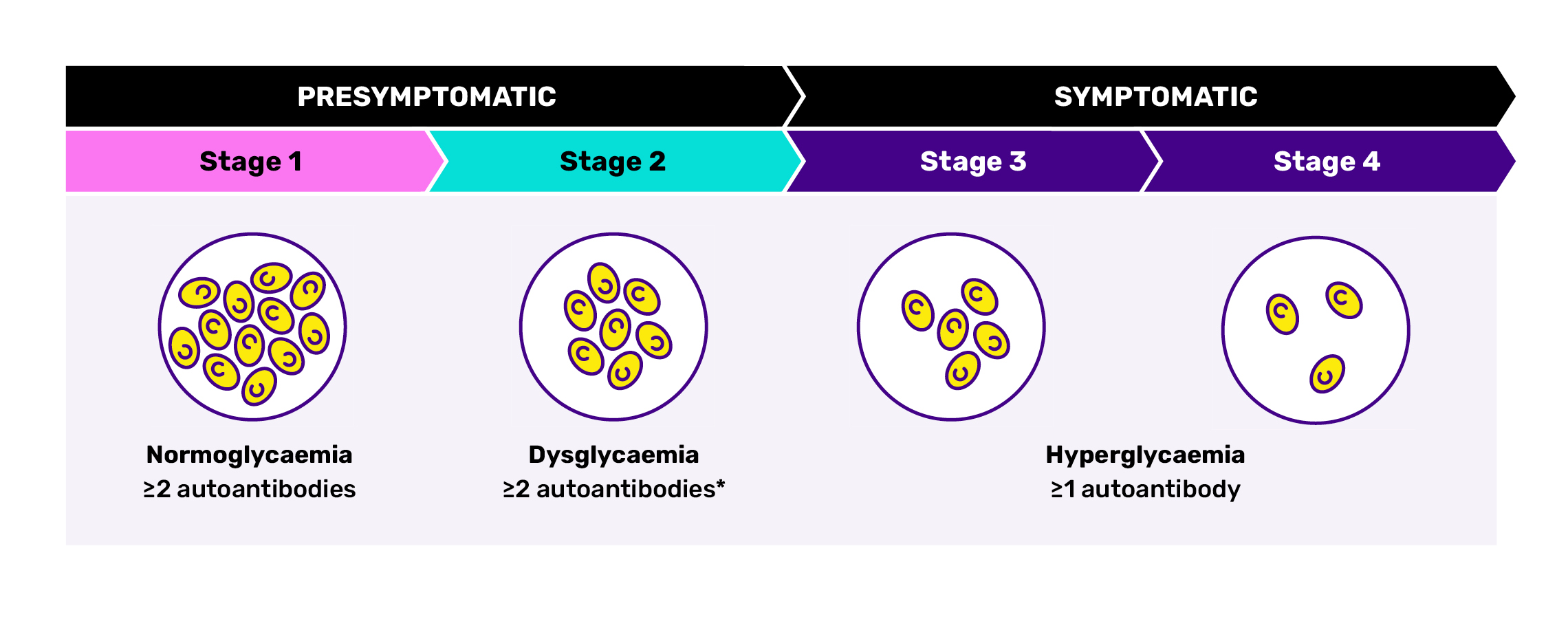
Adapted from Breakthrough T1D. The stages of type 1 diabetes. Accessed September, 2025. https://jdrf.org.au/stages-of-type-1-diabetes/
*Reversion to single autoantibody or negative status can occur in some people with previously confirmed multiple autoantibody positivity.21
Pathogenesis is driven by autoreactive T cells – primarily CD8+ T cells, supported by CD4+ T cells11,31-34 |
By the time symptoms appear, ~60–90% of beta cells may have already been destroyed*26,35-37 |
*Beta cell mass is highly heterogeneous among patients with autoimmune T1D and may not correspond with severity of clinical presentation.38
Beta cell dysfunction can be detected up to 6 years before symptom onset, accompanying islet autoantibody seroconversion.19,20
Screening for T1D autoantibodies allows detection of beta cell dysfunction before symptoms appear21,22
Screening allows individuals and their families to get ahead of autoimmune T1D, helping21-28:
- prevent diabetic ketoacidosis
- avoid misdiagnosis
- reduce stress at diagnosis
- minimise the risk of long-term complications
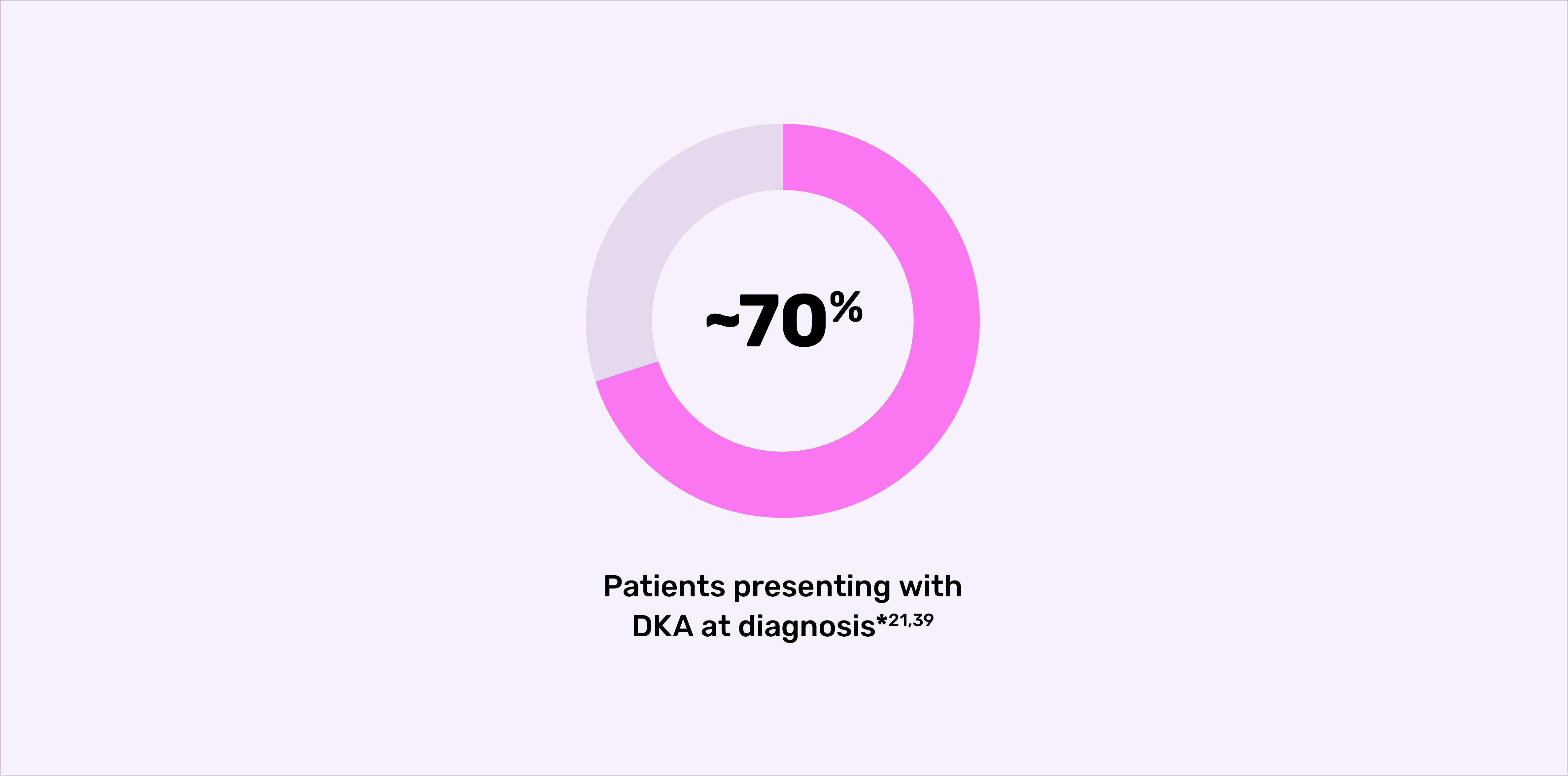
Diabetic ketoacidosis
Up to 70% of patients present with DKA at diagnosis.*21,39
A life-threatening medical emergency,39 DKA is associated with:
- approximately half of all hospitalisations in people with autoimmune T1D40,41
- a sustained negative effect on glycaemic control over time, increasing morbidity and mortality42
- beta cell loss, compounding damage already caused by autoimmunity18,42,43
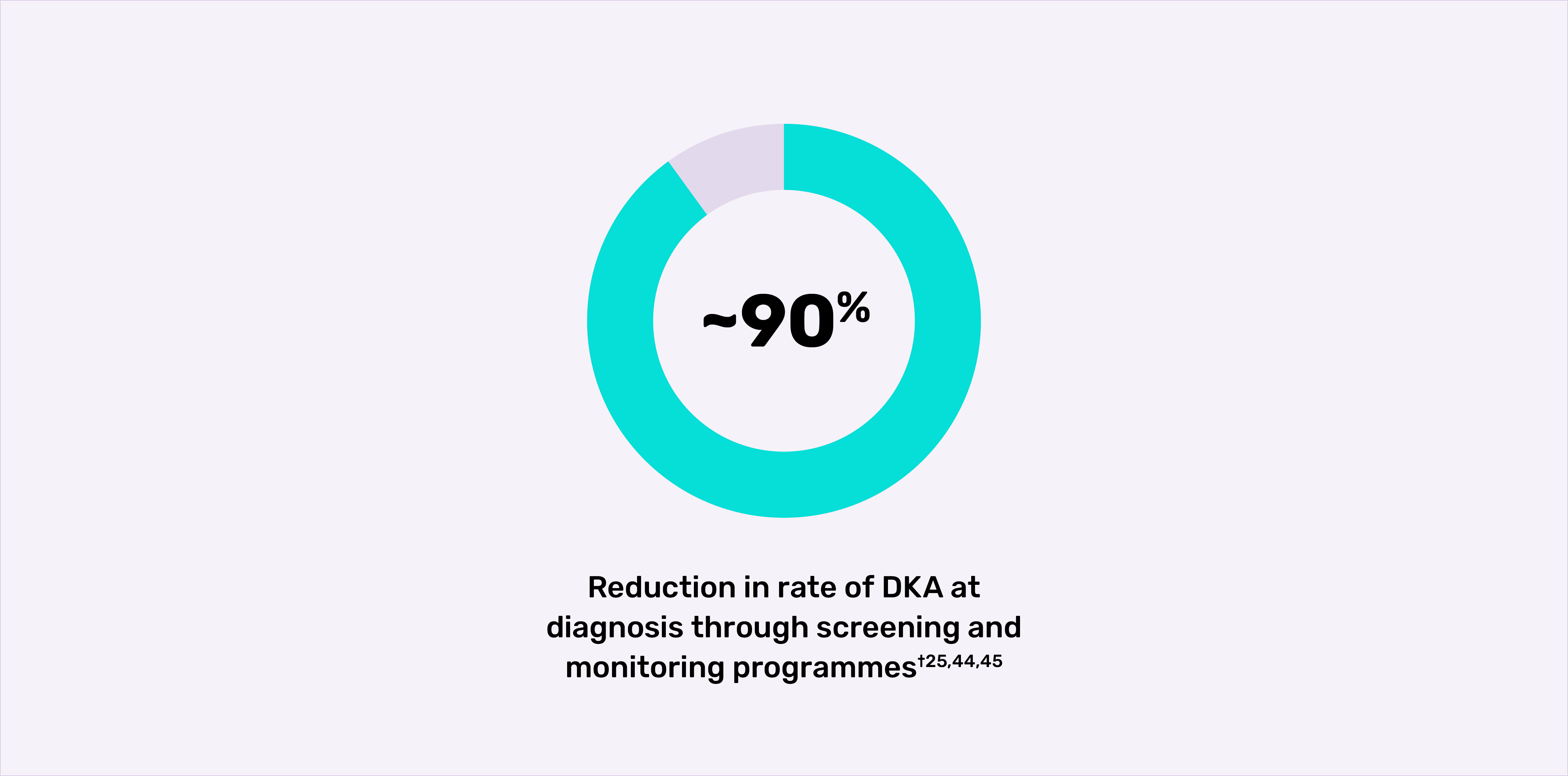
Screening and monitoring programmes have lowered the rate of DKA at diagnosis by as much as 90%.†25,44,45
*Frequencies of DKA range from approximately 15% to 70% across Europe and North America.39
†In a public health screening programme that screened 90,632 children for autoimmune T1D islet autoantibodies in Bavaria, Germany, of the 62 children with presymptomatic autoimmune T1D who developed Stage 3 T1D, two (3.2%) were diagnosed with mild or moderate DKA without clinical symptoms, while 60 (96.8%) did not have DKA. The previously reported prevalence of DKA in unscreened children is over 20% in Germany.44
Beta cells: going beyond insulin
Modern treatment regimens have dramatically improved symptom management, but it is difficult to precisely mimic the complex, multifaceted role beta cells play in tissue health and glycaemic control.12,13,46,47,48
Despite significant advances, achieving glycaemic control remains challenging12,13,49,50
Technological advances have undoubtedly improved the way we manage autoimmune T1D:
- new continuous glucose monitoring (CGM) devices provide patients with glucose readings, trends and alerts in real time13
- automated insulin delivery (AID) systems automatically adjust insulin delivery based on a patient’s glucose readings.50 However, not all patients have access to this technology,51 and a high proportion of patients who do have access still do not achieve their glycaemic targets and continue to experience severe hypoglycaemic episodes.*52
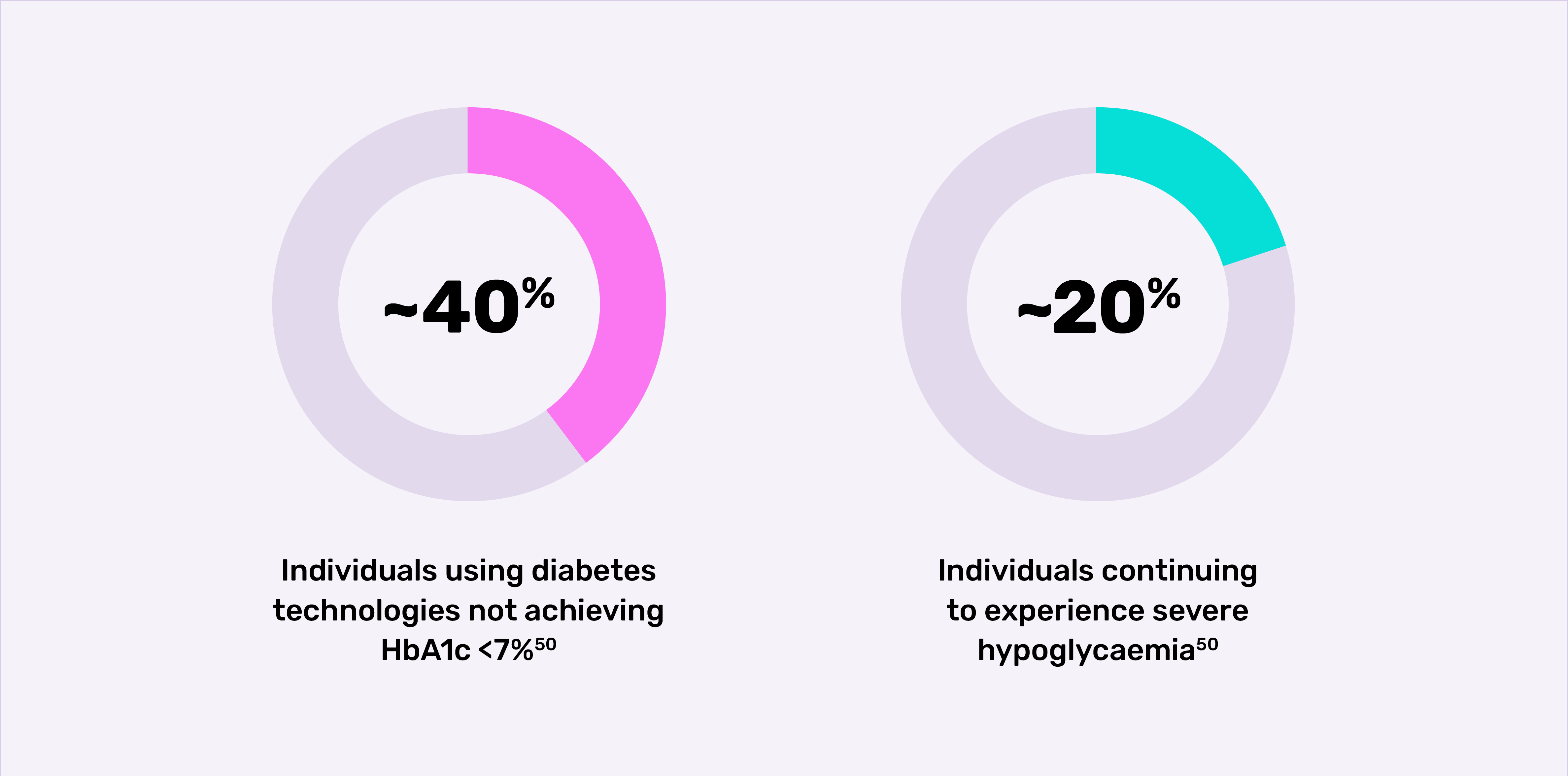
*Retrospective, observational study of 2,074 adults (≥18 years) enrolled from the US T1D Exchange Registry/online community. Participants completed a one-time survey on HbA1c, severe hypoglycaemic episodes and impaired awareness of hypoglycaemia. The majority of participants (91.7%) were using a CGM plus MDI, AID systems or pump. Severe hypoglycaemia was defined as a hypoglycaemic event in the past 12 months where the patient experienced low blood glucose levels that they were unable to treat themselves and needed help from others.50
Conventional exogenous insulin regimens act as a hormone replacement therapy for patients with autoimmune T1D and while they address metabolic symptoms, they do not tackle the underlying autoimmune aetiology of T1D.53,54 |
The next era of T1D management will shift focus from solely symptom control to addressing the underlying immune-mediated cause of the disease11,53-55 |
Beta cells are not only the exclusive producers of insulin46-48
The pancreatic islet is a complex ‘mini organ’ composed of different cell types that work together to integrate systemic and local signals and tightly control blood glucose levels.48,56
As such, beta cells do not exist in isolation, and, beyond insulin, release other hormones, peptides, neurotransmitters and metabolites vital to the complex cellular crosstalk that upholds the overall health and function of the pancreas.47,48,56-59
Beyond insulin, signalling molecules released by beta cells include:
|
Urocortin 3 |
GABA |
Amylin |
Serotonin |
VEGF |
|
Promote somatostatin release, which in turn regulates both beta and alpha cell activity |
Suppresses glucagon release, delays the rate of gastric emptying, promotes satiety |
Increases beta cell mass during metabolic challenge; suppresses glucagon release |
Stimulates blood vessel growth | |
Beta cells also display remarkable functional heterogeneity, differing in their insulin secretion capacity and responsiveness to glucose and other stimuli, and allowing for precise, adaptable and robust glucose regulation.46,57
The issue in autoimmune T1D isn’t just the loss of insulin – it’s the loss of beta cells and islet ability to sense and respond dynamically to the body’s needs.14,62-64 |
C-peptide: a measure of beta cell function
Produced in a 1:1 molecular ratio with insulin, C-peptide is a direct, quantitative and reliable measure of beta cell function.6,16,19,65
C-peptide is a more reliable measure of beta cell function than endogenous insulin because it19,65:
- has a slower rate of degradation
- has a less variable rate of clearance
- is not impacted by exogenous insulin
C-peptide is being increasingly recognised for its utility across clinical and research settings. In clinical practice, it can be used to differentiate between types of diabetes, and growing evidence suggests it may also help predict future level of glycaemic control and risk of complications.65-67 In research settings, C-peptide is serving as a valuable biomarker for assessing response to immunomodulatory treatment.6,68
The impact of residual beta cell function
The link between beta cell activity and clinical outcomes offers a strong, practical rationale for valuing and monitoring beta cell function in the management of autoimmune T1D.1-6,12-17,19
Greater residual beta cell activity is linked to improved outcomes1-6,12-17,19
The pancreatic beta cell reserve determines the course of the autoimmune T1D journey1-7 with higher versus lower residual beta cell activity, as indicated by C-peptide, associated with:
1. Improved glycaemic control
Significantly improved parameters of daily glycaemic control have been observed in individuals with higher versus lower residual beta cell activity, as indicated by level of C-peptide.
Specifically, greater residual beta cell function correlates with*15:
longer time in range
lower HbA1c
lower total daily insulin dose
Time in range by level of C-peptide*15
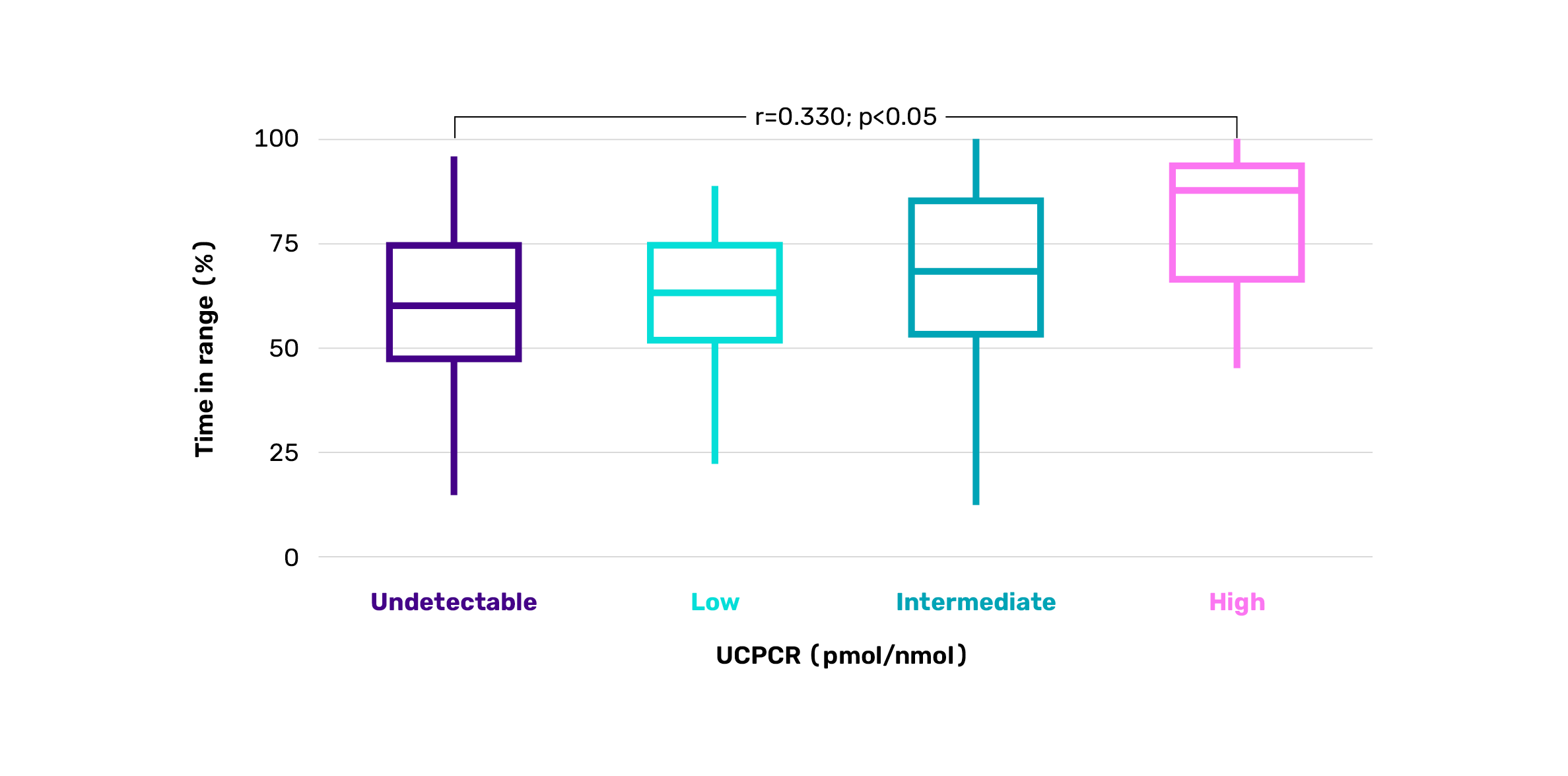
Adapted from Snethlage CMF, et al. Diabetes Care. 2024;47:1114-1121.
*Cross-sectional cohort study of 489 Dutch adults with autoimmune T1D (median duration 15 years) assessing associations between residual beta cell function (measured by UCPCR), alpha cell function (glucagon/glucose ratio) and CGM metrics, including time in range, variability and hypoglycaemia. A higher UCPCR correlated with a longer time in range (r=0.330, p<0.05), and correlated negatively with both HbA1c levels (r= –0.183, p<0.05) and total daily insulin dose (r= –0.183, p<0.05). UCPCR values: undetectable: <0.01 nmol/mmol; low: 0.01–0.2 nmol/mmol; intermediate: 0.2–0.6 nmol/mmol; high: >0.6 nmol/mmol.15
2. Reduced incidence of severe hypoglycaemia
Incidence of severe hypoglycaemia is significantly associated with level of residual beta cell function, as indicated by level of C-peptide.4†
Proportion of patients with a history of severe hypoglycaemia†‡4
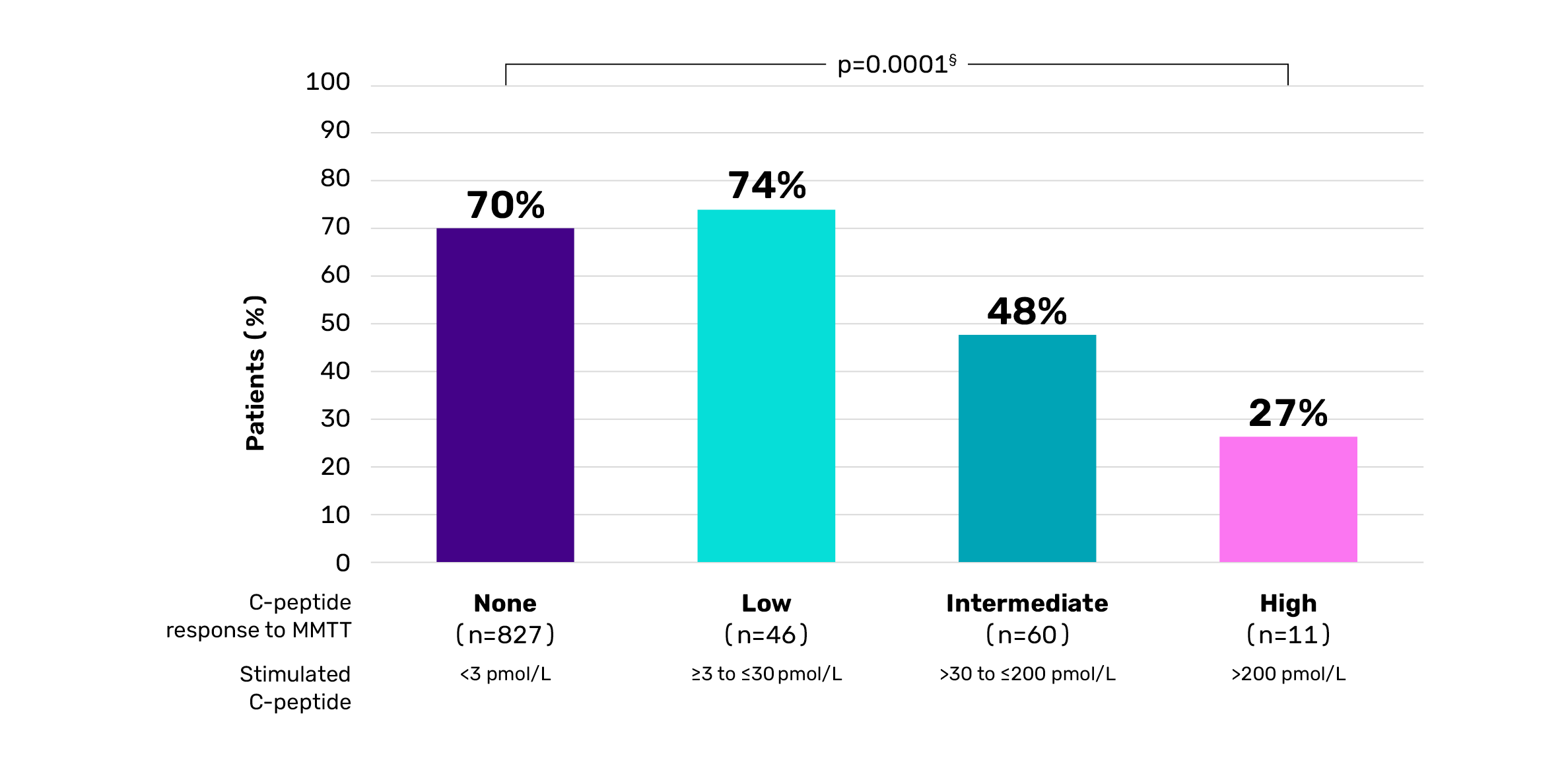
Adapted from Gubitosi-Klug RA, et al. J Clin Invest. 2021;131(3):e143011.
†Observational study of the DCCT/EDIC cohort (mean duration of T1D ~35 years). Serum C-peptide was measured during a 4-hour MMT. Associations with metabolic outcomes and complications were explored among non-responders (all C-peptide values after meal <3 pmol/L) and three categories of responders.4 ‡Hypoglycaemia requiring assistance.4 §Based on Cochran-Armitage trend test.4
3. Reduced likelihood of DKA
Patients with higher versus lower residual beta cell activity are significantly less likely to experience DKA both at the time of diagnosis and 12 months after diagnosis.II18
Longitudinal C-peptide levels according to DKA status at time of diagnosisII18
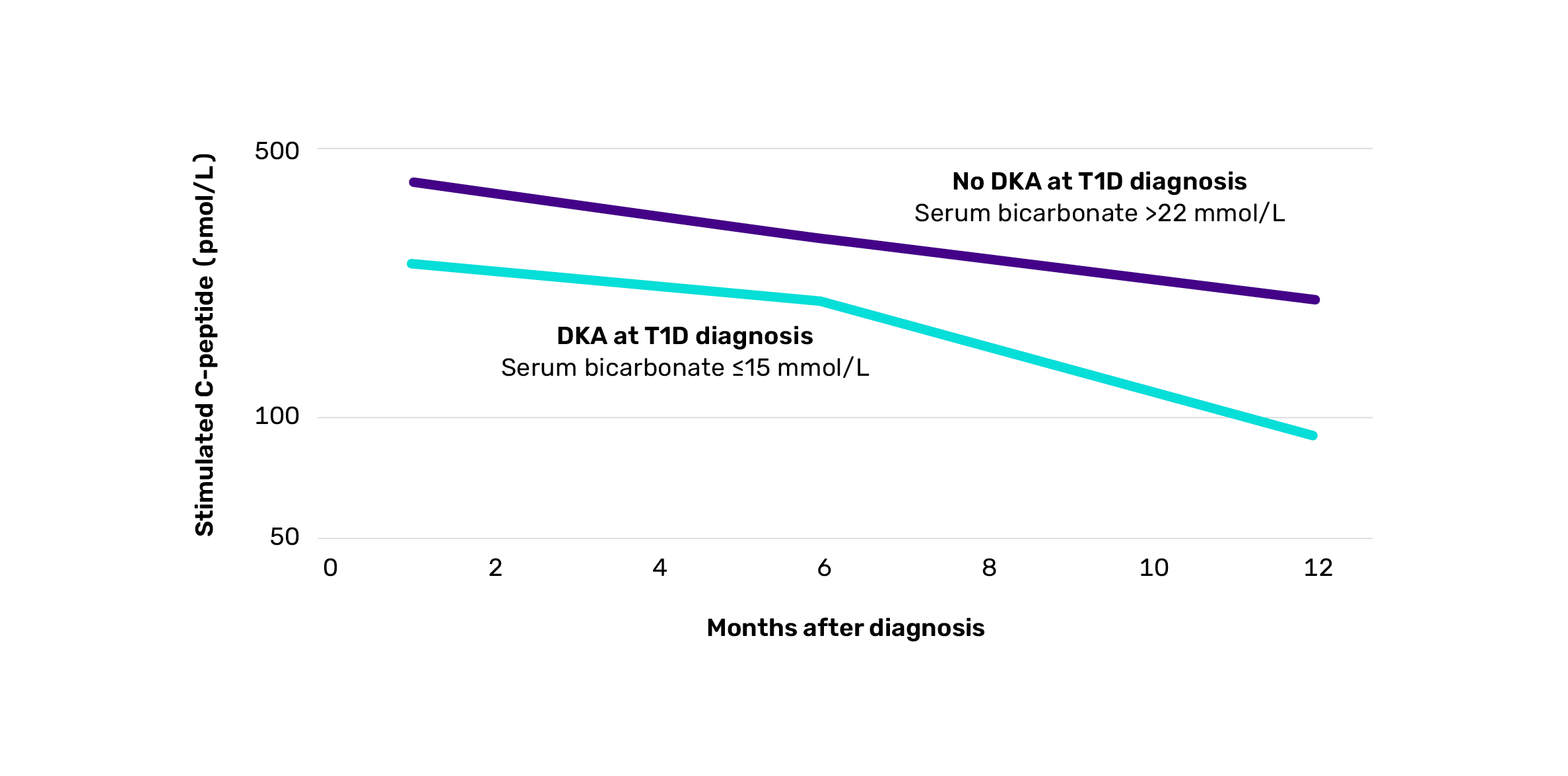
Adapted from Mortensen HB, et al. Pediatr Diabetes. 2010;11(4):218-226.
IIProspective, observational cohort study of 275 children with newly diagnosed T1D conducted across 18 centres in Europe and Japan, assessing predictors of residual beta cell function and glycaemic control over the first 12 months post-diagnosis using serial mixed-meal C-peptide tests, autoantibody levels, HLA typing, and HbA1c measurements. DKA with standard bicarbonate <15 mmol/L was associated with significantly poorer residual beta cell function 1 (p=0.004) and 12 months (p=0.0003) after diagnosis.18
4. Reduced risk of microvascular complications
Patients with higher versus lower residual beta cell activity are at lower risk of long-term microvascular complications such as:
retinopathy5,6,16
nephropathy5
Even relatively low residual beta cell activity correlates with an increased likelihood of achieving recommended HbA1c targets and a reduced prevalence of severe hypoglycaemia2,4
Those with low residual beta cell function are still 2x more likely to achieve HbA1c <7.5% compared to patients with no residual beta cell function.¶2
Patients with a HbA1c <7.5% by level of C-peptide¶
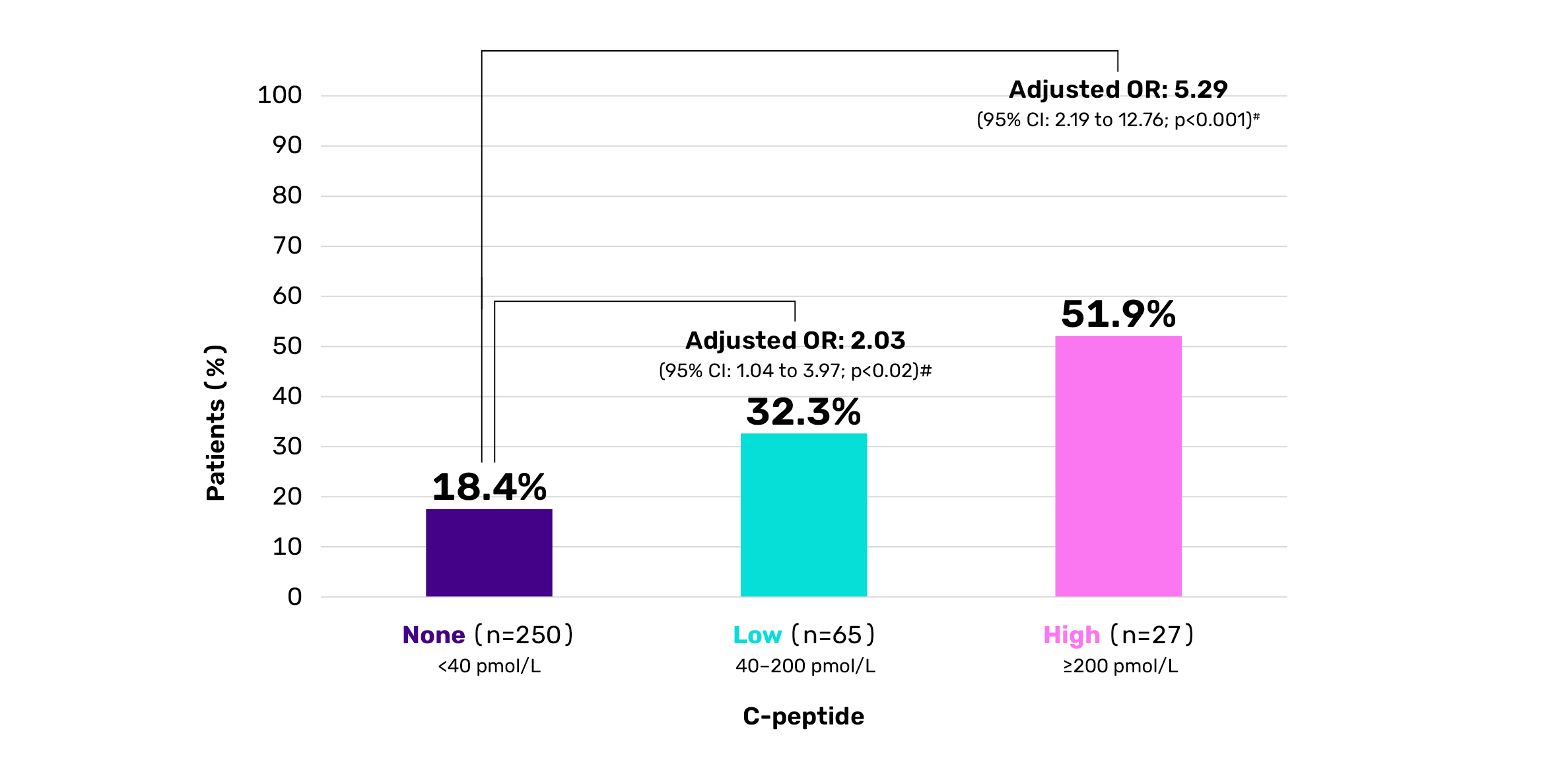
¶Cross-sectional study of 342 Danish children (aged 4.8–18.9 years) with autoimmune T1D (duration 3–6 years) assessing residual beta cell function via meal- stimulated C-peptide and evaluating associations with hypoglycaemia, HbA1c and insulin needs. C-peptide levels: none: <40 pmol/L; low: 40–200 pmol/L; high: ≥200 pmol/L.2 #Logistic regression analysis adjusted for sex, age, pubertal status, diabetes duration, and insulin administration technique.2
Relatively low residual beta cell activity is also associated with a protective effect against hypoglycaemia. In the DCCT, C-peptide levels above the detection threshold of 30 pmol/L were significantly associated with a lower prevalence of severe hypoglycaemia throughout the entire study period.4
Summary
The more beta cells the better, and even limited residual activity can still positively impact the patient journey1-6,15-17,19
- In symptomatic autoimmune T1D, clinically meaningful beta cell function is indicated by a C-peptide level over 0.2 pmol/mL7
- But improved outcomes are still observed with any level above 0.03 pmol/mL (detection threshold)2,4-7

Abbreviations
AID, automated insulin delivery system; CGM, continuous glucose monitor; DanDiabKids, Danish Study Group for Childhood Diabetes; DKA, diabetic ketoacidosis; DCCT, Diabetes Control and Complications Trial; EDIC, Epidemiology of Diabetes Interventions and Complications; GABA, gamma-aminobutyric acid; HbA1c, haemoglobin A1c; MDI, multiple daily injections; MMTT, mixed meal tolerance test; SDRNT1BIO, Scottish Diabetes Research Network Type 1 Bioresource Study; T1D, type 1 diabetes; UCPCR, urinary C-peptide-to-creatine ratio; US, United States; VEGF, vascular endothelial growth factor.
References
- Steffes MW, Sibley S, Jackson M, et al. Diabetes Care. 2003;26(3):832-836.
- Sørensen JS, Johannesen J, Pociot F, et al. Diabetes Care. 2013;36(11):3454-3459.
- Jeyam A, Colhoun H, McGurnaghan S, et al. Diabetes Care. 2021;44(2):390-398.
- Gubitosi-Klug RA, Braffett BH, Hitt S, et al. J Clin Invest. 2021;131(3):e143011.
- Lachin JM, McGee P, Palmer JP; DCCT/EDIC Research Group. Diabetes. 2014;63(2):739-748.
- Latres E, Greenbaum CJ, Oyaski ML, et al. Diabetes. 2024;73(6):823-833.
- Flatt AJS, Greenbaum CJ, Shaw JAM, et al. Ann N Y Acad Sci. 2021;1495(1):40-54.
- Herold KC, Delong T, Perdigoto AL, et al. Nat Rev Immunol. 2024;24(6):435-451.
- O’Donovan AJ, Gorelik S, Nally LM. Front Endocrinol (Lausanne). 2024;15:1477101.
- Ozen G, Zanfardino A, Confetto S, et al. Int J Endocrinol. 2020;2020:2630827.
- Nagy G, Szekely TE, Somogyi A, et al. World J Diabetes. 2022;13(10):835-850.
- Kramer CK, Retnakaran R, Zinman B. Cell Metab. 2021;33(4):740-747.
- Laffel LM, Kanapka LG, Beck RW, et al. JAMA. 2020;323(23):2388-2396.
- Aronoff SL, Berkowitz K, Schreiner B, et al. Diabetes Spectr. 2004;17(3):183-190.
- Snethlage CMF, McDonald TJ, Oram RD, et al. Diabetes Care. 2024;47(7):1114-1121.
- Palmer JP, Fleming GA, Greenbaum CJ, et al. Diabetes. 2004;53(1):250-264.
- Nathan DM. Diabetologia. 2021;64(5):1049-1058.
- Mortensen HB, Swift PGF, Holl RW, et al. Pediatr Diabetes. 2010;11(4):218-226.
- Galderisi A, Carr ALJ, Martino M, et al. Diabetologia. 2023;66(12):2189-2199.
- Koskinen MK, Helminen O, Matomäki J, et al. Eur J Endocrinol. 2016;174(3):251-259.
- Phillip M, Achenbach P, Addala A, et al. Diabetes Care. 2024;47(8):1276-1298.
- Moore DJ, Leibel NI, Polonsky W, et al. Int J Gen Med. 2024;17:3003-3014.
- Haller MJ, Bell KJ, Besser REJ et al. Horm Res Paediatr. 2024;97(6):529-545.
- Narendran P. Diabetologia. 2019;62(1):24-27.
- Besser REJ, Ng SM, Gregory JW, et al. Arch Dis Child. 2022;107(9):790-795.
- Breakthrough T1D. Early detection: How type 1 diabetes screening can change lives. Accessed September, 2025. https://breakthrought1d.org.uk/resources/early-detection-how-type-1-diabetes-screening-can-change-lives/
- Quinn LM, Rashid R, Narendran P, et al. Br J Gen Pract. 2022;73(726):36-39.
- Fowler MJ. Clin Diabetes. 2008;26(2):77-82.
- Sims EK, Besser REJ, Dayan C, et al. Diabetes. 2022;71(4):610-623.
- Breakthrough T1D (formerly JDRF). The stages of type 1 diabetes. Accessed September, 2025. https://jdrf.org.au/stages-of-type-1-diabetes/
- Burrack AL, Martinov T, Fife BT. Front Endocrinol (Lausanne). 2017;8:343.
- Addissouky TA, Ali MMA, El Sayed IET, et al. Bull Natl Res Cent. 2024:48:42.
- Houeiss P, Luce S, Boitard C. Front Endocrinol (Lausanne). 2022;13:933965.
- Pugliese A. Pediatr Diabetes. 2016;17(Suppl 22):31-36.
- Gitelman SE, Evans-Molina C, Guolo A, et al. Diabetes. 2023;72(9):1289-1296.
- Kawasaki E. Int J Mol Sci. 2023;24(12):10012.
- Wang YN, Li R, Huang Y, et al. Front Immunol. 2024;15:1450366.
- Oram RA, Sims EK, Evans-Molina C. Diabetologia. 2019;62(4):567-577.
- Wolfsdorf JI, Glaser N, Agus M, et al. Pediatr Diabetes. 2018;19(Suppl 27):155-177.
- Tomic D, Craig ME, Magliano DJ, et al. Diabet Med. 2024;41(1):e15218.
- AbuHammad GAR, Naser AY, Hassouneh LKM. BMC Endocr Disord. 2023;23(1):102.
- Duca LM, Wang B, Rewers M, et al. Diabetes Care. 2017;40(9):1249-1255.
- Castañer MF, Montaña E, Camps I, et al. Diabetes Metab. 1996;22(5):349-355.
- Ziegler AG, Kick K, Bonifacio E, et al. JAMA. 2020;323(4):339-351.
- Larsson HE, Vehik K, Bell R, et al. Diabetes Care. 2011;34(11):2347-2352.
- Toren E, Burnette KS, Banerjee RR, et al. Front Immunol. 2021;12:756548.
- Almaça J, Caicedo A, Landsman L. Diabetologia. 2020;63(10):2076-2085.
- Noguchi GM & Huising MO. Nat Metab. 2019;1(12):1189-1201.
- Holt RIG, DeVries JH, Hess-Fischl A, et al. Diabetes Care. 2021;44(11):2589-2625.
- Sherr JL, Laffel LM, Liu J, et al. Diabetes Care. 2024;47(6):941-947.
- Lacy ME, Lee KE, Atac O, et al. Clin Diabetes. 2024;42(3):388-3975
- Sherr JL, Heinemann L, Fleming GA, et al. Diabetologia. 2023;66(1):3-22.
- Singh A, Afshan N, Singh A, et al. Eur J Cell Biol. 2023;102(2):151329.
- Ajmal N, Bogart MC, Khan P, et al. Cells. 2023;12(11):1472.
- Hankosky ER, Schapiro D, Gunn KB, et al. Diabetes Ther. 2023;14(6):967-975.
- Walker JT, Saunders D, Brissova M, et al. Endocr Rev. 2021;42(5):605-657.
- Benninger RKP & Kravets V. Nat Rev Endocrinol. 2021;18(1):9-22.
- Hartig SM & Cox AR. J Mol Med (Berl). 2020;98(4):451-467.
- Langlois A, Dumond A, Vion J, et al. Front Endocrinol (Lausanne). 2022;13:836344.
- Zhang XX, Pan YH, Huang YM, et al. World J Diabetes. 2016;7(9):189-197.
- Almaça J, Molina J, Menegaz D, et al. Cell Rep. 2016;17(12):3281-3291.
- Podobnik B, Koroŝak D, Klemen MS, et al. Biophys J. 2020;118(10):2588-2595.
- Hoang D-T, Hara M, Jo J. PLoS One. 2016;11(4):e0152446.
- Hill TG & Hill DJ. Int J Mol Sci. 2024;25(7):4070.
- Leighton E, Sainsbury CAR, Jones GC. Diabetes Ther. 2017;8(3):475-487.
- Maddaloni E, Bolli GB, Frier BM, et al. Diabetes Obes Metab. 2022;24(10):1912-1926.
- Jones AG & Hattersley AT. Diabet Med. 2013;30(7):803-817.
- Taylor PN, Collins KS, Lam A, et al. Lancet Diabetes Endocrinol. 2023;11(12):915-925.
MAT-GLB-2502461-V1.0-10/2025