- Article
- Source: Campus Sanofi
- 17 May 2024
Global reach of over 20 years experience in the patient-centered Fabry Registry
Design of the Fabry Registry and objectives
- The Fabry Registry is a multicenter, international, longitudinal, observational study open to all patients with a confirmed diagnosis of FD, irrespective of treatment status or therapy administered
- It is designed to collect consistent retrospective and prospective patient data with the primary objectives of exploring and defining the variability in the natural history and clinical characteristics across the disease spectrum, and of tracking and characterizing long-term treatment outcomes in a real-world setting
Enrollment of patients in the Fabry Registry over time
- The Fabry Registry has grown significantly from an initial enrollment of 21 patients with FD at two sites in 2001 to 8,098 patients (43.5% male, 56.5% female) as of October 7, 2022
- The Fabry Registry has a global reach, with a current presence in 44 countries worldwide and 219 participating sites (EMEA 45.7%; North America 40.3%; JAPAC 8.1%; Latin America 5.9%)
- Genotype data are available for 90.3% of patients and, overall, 1,039 GLA variants are represented in the Fabry Registry: classic phenotype (406 variants, 3,532 patients), later-onset phenotype (54 variants, 1,239 patients), and “other” phenotypes* (e.g. 70 variants, 762 patients)
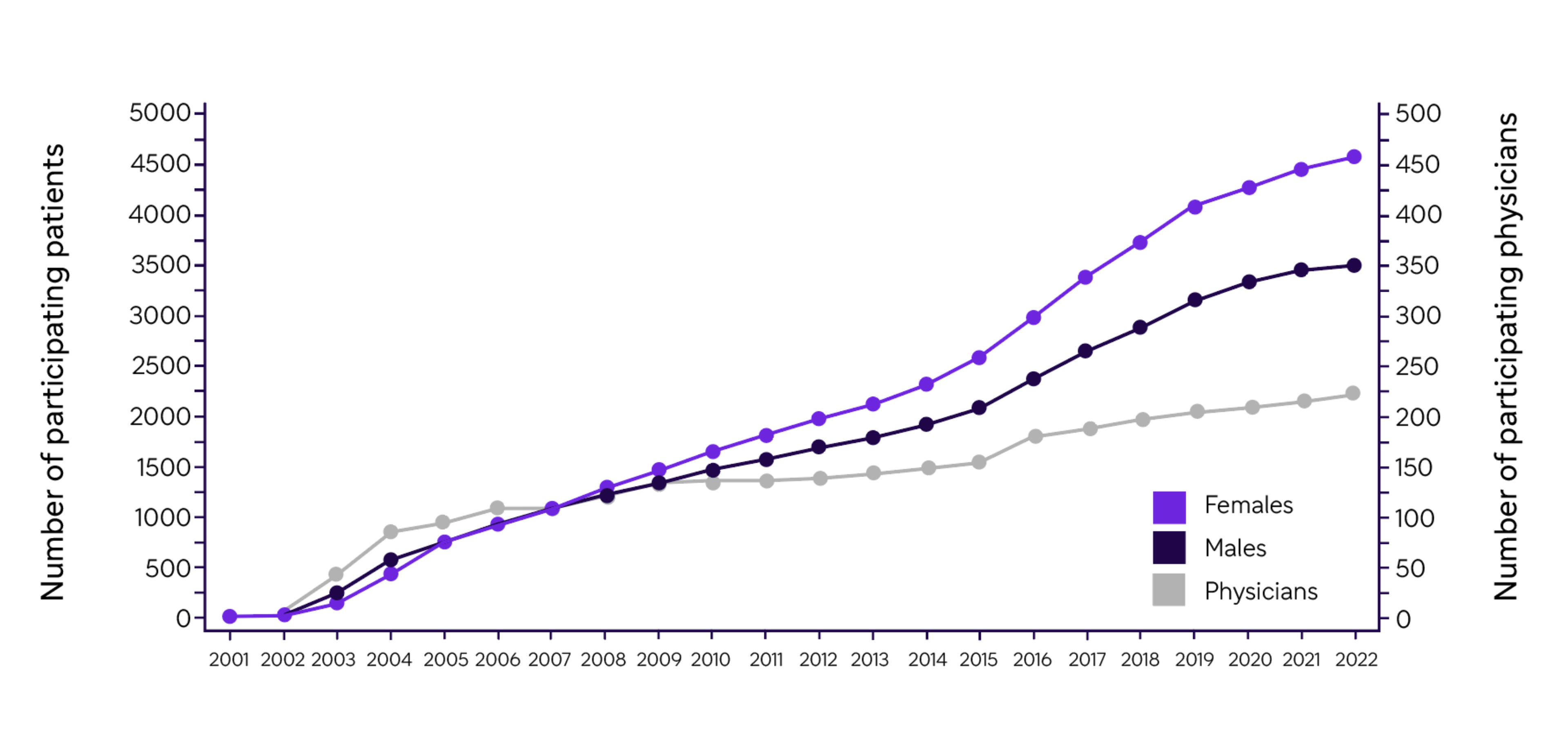
Cumulative numbers of participating physicians and patients enrolled in the Fabry Registry by year of enrollment
During the natural history period, patients in the Fabry Registry had a total of 311,137 person-years from birth to last follow-up**
(122,599 years for males, 188,537 years for females).
Treatment time observed amounts to a total of 40,419 person-years among “ever-treated” patients
(23,983 years for males, 16,436 years for females)
During the natural history period, patients in the Fabry Registry had a total of 311,137 person-years from birth to last follow-up**
(122,599 years for males, 188,537 years for females).
Treatment time observed amounts to a total of 40,419 person-years among “ever-treated” patients
(23,983 years for males, 16,436 years for females)
* [Likely] benign, likely classic, likely later-onset, variants of uncertain significance.
**Date of treatment initiation (for “ever-treated” patients) or date of most recent data entry (for “never-treated” patients).
Topics covered by Fabry Registry publications
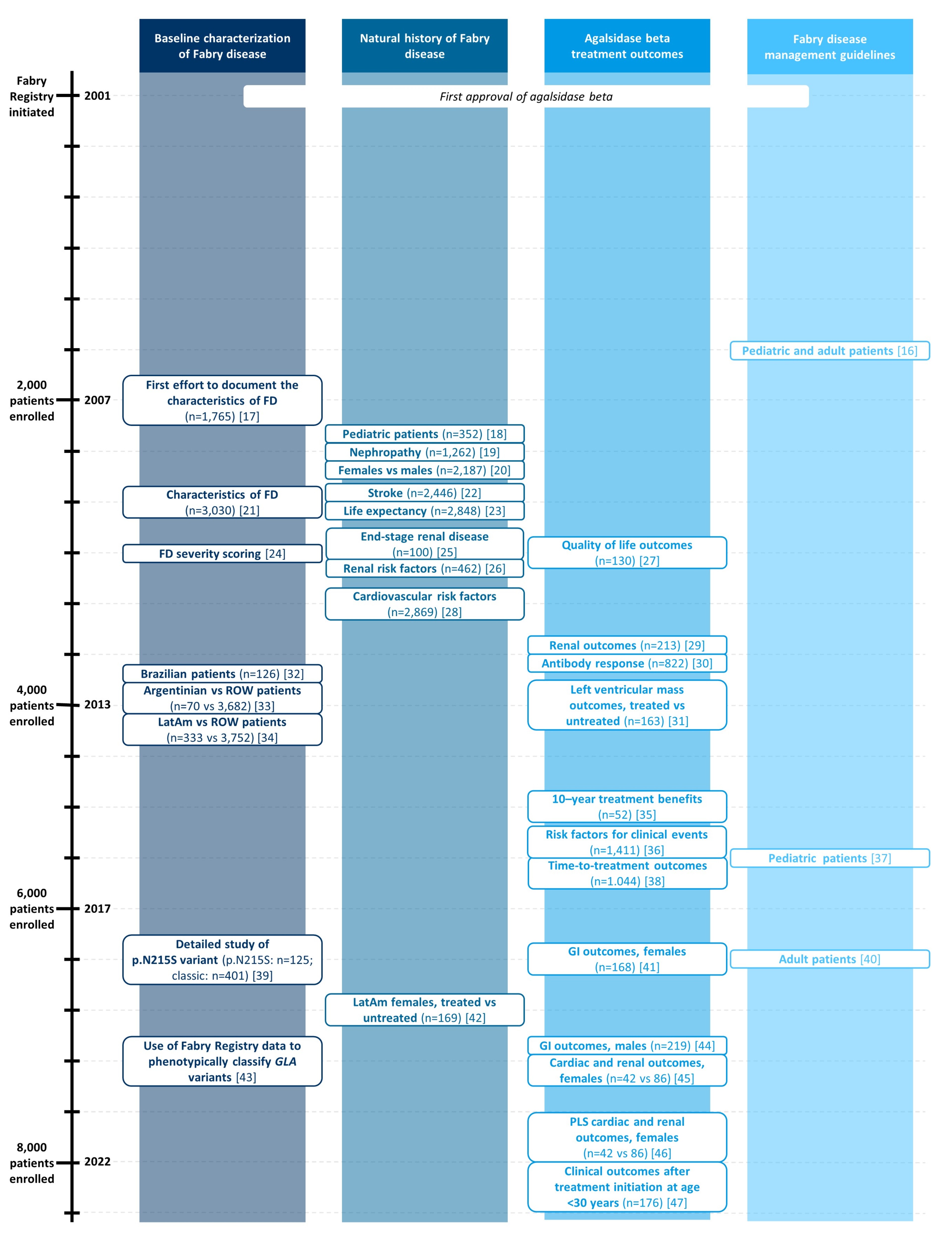
Key contributions from 20+ years of the Fabry Registry
- The Fabry Registry has now been operational for over 20 years and has collected real-world demographic and longitudinal clinical data from more than 8,000 individuals with FD
- Leveraging the accumulating evidence base, multidisciplinary collaborations have resulted in the creation of 32 peer-reviewed scientific publications on the onset and progression of FD, its clinical management, the role of sex and genetics, the outcomes of ERT with Fabrazyme®, and prognostic factors
|
T. Watt, et al. Genet. Med. (2010) |
Agalsidase beta treatment is associated with improved quality of life in patients with Fabry disease: findings from the Fabry Registry |
|
D.G. Warnock, et al. Nephrol. Dial. Transplant. (2012) |
Renal outcomes of agalsidase beta treatment for Fabry disease: role of proteinuria and timing of treatment initiation: findings from the Fabry Registry |
|
W.R. Wilcox, et al. Mol. Genet. Metab. (2012) |
Anti-alpha-galactosidase A antibody response to agalsidase beta treatment: data from the Fabry Registry |
|
D.P. Germain, et al. Genet. Med. (2013) |
Ten-year outcome of enzyme replacement therapy with agalsidase beta in patients with Fabry disease |
|
D.P. Germain, et al. J. Med. Genet. (2015) |
Ten-year outcome of enzyme replacement therapy with agalsidase beta in patients with Fabry disease |
|
A. Ortiz, et al. J. Med. Genet. (2016) |
Time to treatment benefit for adult patients with Fabry disease receiving agalsidase-ß: data from the Fabry Registry |
|
R.J. Hopkin, et al. Mol. Genet. Metab. (2016) |
Risk factors for severe clinical events in male and female patients with Fabry disease treated with agalsidase beta enzyme replacement therapy: data from the Fabry Registry |
|
W.R. Wilcox, et al. JIMD Rep. (2018) |
Improvement of Fabry disease-related gastrointestinal symptoms in a significant proportion of female patients treated with agalsidase beta: data from the Fabry Registry |
|
R.J. Hopkin, et al. Mol. Genet. Metab. Rep. (2020) |
Improvement of gastrointestinal symptoms in a significant proportion of male patients with classic Fabry disease treated with agalsidase beta: a Fabry Registry analysis stratified by phenotype |
|
C. Wanner, et al. E.S.C. Heart Fail. (2020) |
Cardiomyopathy and kidney function in agalsidase beta treated female Fabry patients: a pre‑treatment vs post-treatment analysis |
|
C. Wanner, et al. Future Cardiol. (2022) |
Plain language summary of a study looking at heart muscle thickness and kidney function in women with Fabry disease who received agalsidase beta treatment |
|
R.J. Hopkin, et al. Mol. Genet. Metab. (2023) |
Clinical outcomes among young patients with Fabry disease who initiated agalsidase beta treatment before 30 years of age: an analysis from the Fabry Registry |
- Since inception in 2001, the Fabry Registry has evolved from paper case-report forms to a state-of-the-art, interactive, mobile-friendly, web-based collection and reporting technology platform RegistryNXT!
- The Fabry Registry has been evolving and adapting appropriately, and relevant changes have been implemented as deemed necessary over the years:
- New cardiac MRI and other cardiovascular and cerebrovascular parameters have been included to reflect the advancement of imaging technology
- Plasma lyso-GL-3 data are collected since 2017 as the body of evidence supporting its potential value as a biomarker for disease staging and treatment response
- Following the outbreak of the COVID-19 pandemic, questions about the potential susceptibility of FD patients and impacts on disease outcomes prompted the development of the COVID-19 case-report form in 2020
- In the future, the potential benefit of newborn screening to the understanding of FD would be greatly limited without focused registry involvement in this rapidly emerging area
- Continued collection of longitudinal data expands opportunities to apply advanced statistical methodologic approaches that are commonly used for analysis of more prevalent diseases:
- Stratification and/or subgrouping of patients based on important patient demographics, genotype, and baseline disease severity to evaluate and control for confounding
- Separating samples into subgroups according to FD phenotype or other potential confounders is of critical importance to discerning differences in treatment outcomes
- To overcome the lack of appropriate untreated comparison groups, the innovative self-controlled pre-versus post-treatment comparison approach to the analysis of clinical outcome data from patients with FD was implemented
Use of real-world data in global multistakeholder collaborations
- Fabry Registry analyses are used in multistakeholder engagement between healthcare professionals, patients and patient organizations, payors, and healthcare policy makers, and can support decision making by regulatory agencies
- The patient-centered Fabry Registry fosters collaborative research partnerships with the overarching goal of optimizing the clinical management of patients with FD and is well positioned to add to its past achievements
Learn more about Fabrazyme®

Fabrazyme® evidence
Explore the results and evidence supporting Fabrazyme® and how it was studied through several clinical trials.

Fabrazyme® safety profile
Find out more about the safety and tolerability profile for Fabrazyme®.

Fabrazyme® evidence
Explore the results and evidence supporting Fabrazyme® and how it was studied through several clinical trials.

Fabrazyme® safety profile
Find out more about the safety and tolerability profile for Fabrazyme®.
EMEA, Europe Middle-East and Africa; ERT, enzyme replacement therapy; FD, Fabry disease; GI, gastrointestinal; JAPAC, Japan and Asia-Pacific; LatAm, Latin America; lyso-GL-3, globotriaosylsphingosine; MRI, magnetic resonance imaging; PLS, plain language summary publication; ROW, rest of the world.
MAT-XU-2402117 (v1.0) Date of preparation: May 2024