- Article
- Source: Campus Sanofi
- 23 Oct 2023
Understanding the impact of Fabrazyme (agalsidase beta) on long-term renal function

Fabrazyme® (agalsidase beta) prescribing information GB
Fabrazyme® (agalsidase beta) prescribing information NI
In an open-label, phase III extension study, early initiation of Fabrazyme was shown to maintain renal function over 54 months1
Background
- A 54-month observational study of Fabrazyme in 58 Classic Fabry patients (56 male, 2 female) with a mean age of 31 years at baseline showed:1
- Median eGFR (-1.0mL/min per 1.73 m2/yr) was maintaned within the normal range over 54 months
- Patients without significant renal involvment (proteinuria ≤ 1g/day) (n=42) at baseline benefited the most
- Median eGFR (-1.0mL/min per 1.73 m2/yr) was maintaned within the normal range over 54 months
Key data
Sustained, long-term renal stabilisation (media eGFR) after 54 months of Fabrazyme therapy in patients with Fabry disease.
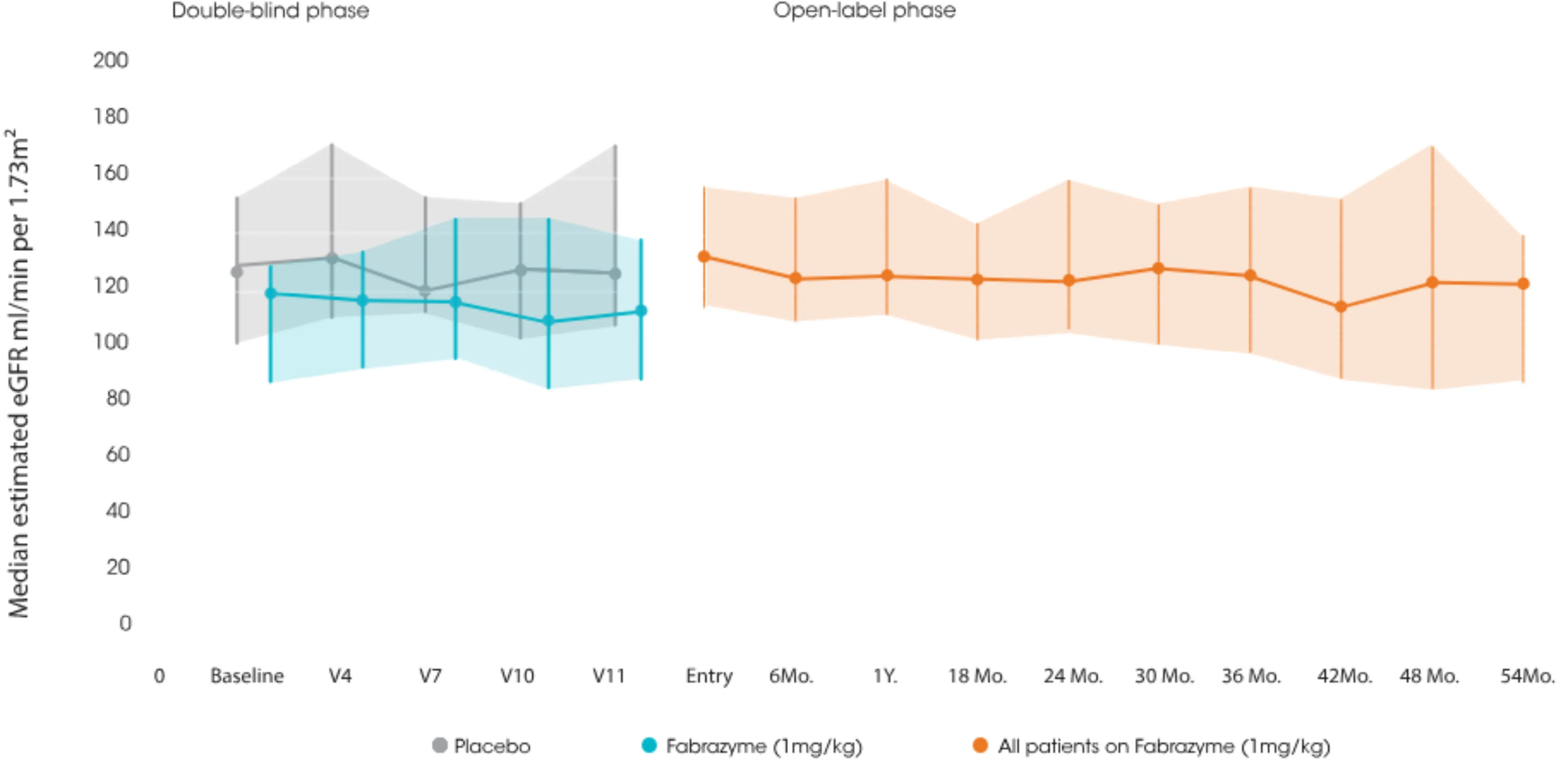
Adapted from Germain DP et al. Am Soc Nephrol. 2007.
Relevance to clinical practice
- Timely initiation of Fabry-specific treatment, such as Fabrazyme, prior to the development of significant organ damage may help to optimise patient outcomes
- Fabry disease is progressive and often life-limiting,2-4 ensuring the long-term efficacy of the selected Fabry-specific therapy is essential
Learn more about Fabrazyme®

Fabrazyme® evidence
Explore the results and evidence supporting Fabrazyme® and how it was studied through several clinical trials.

Fabrazyme® safety profile
Find out more about the safety and tolerability profile for Fabrazyme®.

Fabrazyme® evidence
Explore the results and evidence supporting Fabrazyme® and how it was studied through several clinical trials.

Fabrazyme® safety profile
Find out more about the safety and tolerability profile for Fabrazyme®.
References
- Germain DP et al. J Am Soc Nephrol.2007;18(5);1547-1557.
- Germain DP. J Am Soc Nephrol. 2002;13(suppl 2):S150–S153.
- Desnick RJ, Loannou YA, Eng CM. Chapter 150: α-Galactosidase A Deficiency: Fabry Disease. In: Valle D, Beaudet AL, Vogelstein B, eds. The Online Metabolic and Molecular Basis of Inherited Disease. New York, NY: McGraw Hill; 2014. Available at: https://ommbid.mhmedical.com/content.aspx? sectionid=225546984&bookid=2709 Accessed: January 2022.
- Ortiz A, Germain DP, Desnick RJ, et al. Mol Genet Metab. 2018;123(4):416–427.
MAT-XU-2301503 (v3.0) Date of preparation: May 2024