- Article
- Source: Campus Sanofi
- Feb 28, 2024
Hemophilia B is different. Let’s manage it that way

Explore the differences between factor VIII and
factor IX

Learn more about evaluating multiple pharmacokinetic (PK) parameters

See the additional considerations for the management of hemophilia B
Due to the distinct behavior of factor IX, multiple PK parameters should be considered when assessing bleed prevention. Learn how a broader view of PK may influence evaluation of treatment and management for patients with hemophilia B.1,2
Hemophilia A and hemophilia B are different bleeding disorders with unique pathologies and clinical features3
| Hemophilia A3,4 | |
| Prevalence | 1:5,000 males |
| Clinical symptoms | Joint bleeding, muscle hematoma, soft tissue bleeding |
| Coagulation factor deficiency | Factor VIII |
| Patients with severe factor VIII deficiency | ~50% |
| Annual bleed rate in moderate to severe patients | 14-16 |

| Hemophilia B3,4 | |
| Prevalence | 1:30,000 males |
| Clinical symptoms | Joint bleeding, muscle hematoma, soft tissue bleeding |
| Coagulation factor deficiency | Factor IX |
| Patients with severe factor IX deficiency | ~30% |
| Annual bleed rate in moderate to severe patients | 9-11 |
- Since bleeding phenotype may differ between severe hemophilia A and B, management strategies should be individualized1
Factor VIII and factor IX have unique pharmacokinetic profiles and behave differently in the body1,2,5-7
The 4 key PK differences between factor VIII and factor IX are
- half-life: The time it takes for the concentration of the drug in the plasma to be half of the initial concentration,
- clearance: The ratio of administered dose and area under the curve, showing the amount of plasma free of drug in a given period of time,
- recovery: The increase of coagulation factor activity in the plasma after dose administration
- and volume of distribution: The relationship between the amount of drug in the body and the plasma drug concentration. Drugs with a high volume of distribution will tend to have lower trough levels.
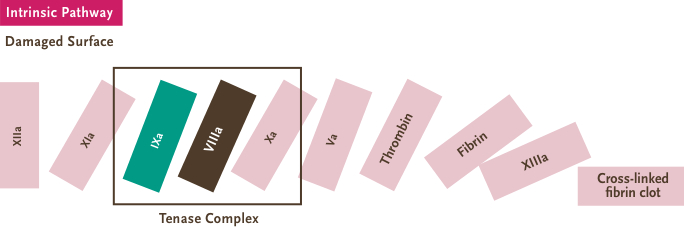
- Factor VIII and factor IX are unique blood-clotting proteins with distinct functions in the coagulation cascade5-7
See a broader view of PK

The unique pharmacokinetic profile of factor IX is explained by its widespread distribution in the extravascular space5,8-10
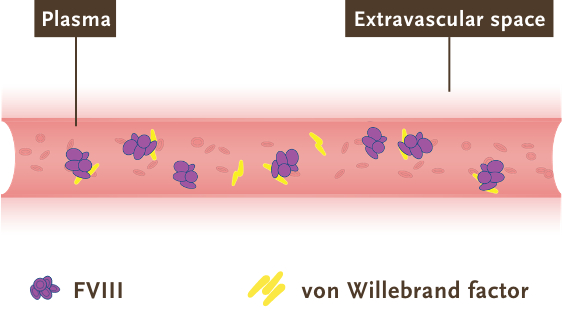
- Factor VIII: largely influenced by its association with von Willebrand factor (vWF), which restricts factor VIII circulation to the bloodstream and limits its extravascular distribution5,12,14
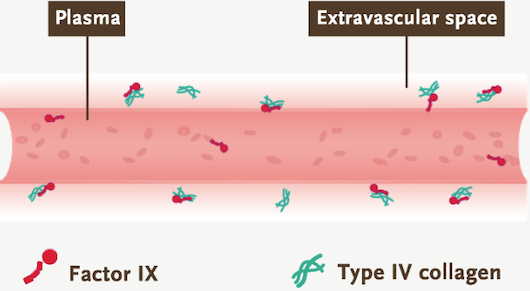
- Factor IX: greatly influenced by its extravascular distribution and type IV collagen binding. The unique distribution behavior of factor IX allows for its longer half-life5,10,15*
*As shown from research of factor IX binding to type IV collagen in human arterial tissue in vitro.
Compartment models are commonly used to describe the PK of therapies such as factor VIII and factor IX5,14
The PK of infused factor VIII may be described by a 2-compartment model5
- After infusion of factor VIII, there is a rapid distribution phase (binding to vWF), followed by elimination. Therefore, its PK may be described by the 2-compartment model5
- However, this early, rapid phase of distribution to vWF rarely contributes to a noticeable difference in the area under the curve (AUC), allowing for an easier PK analysis16
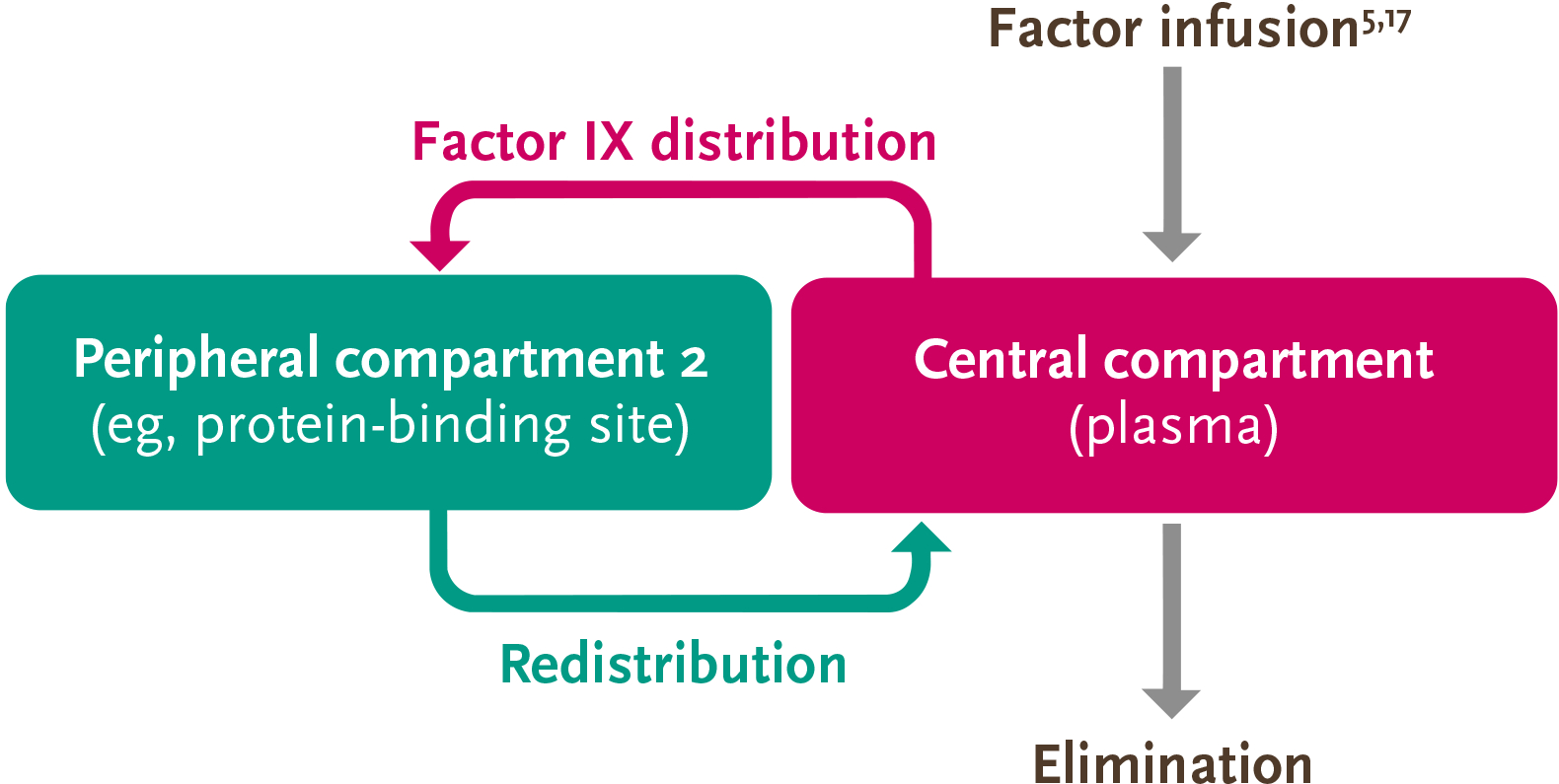
The PK of factor IX can be described by the 3-compartment model, so accurate measurement of its activity is more complex than that of factor VIII5
Due to the distribution of factor IX outside of the plasma and into the extravascular space, the PK behavior is more complex than that of factor VIII5,13
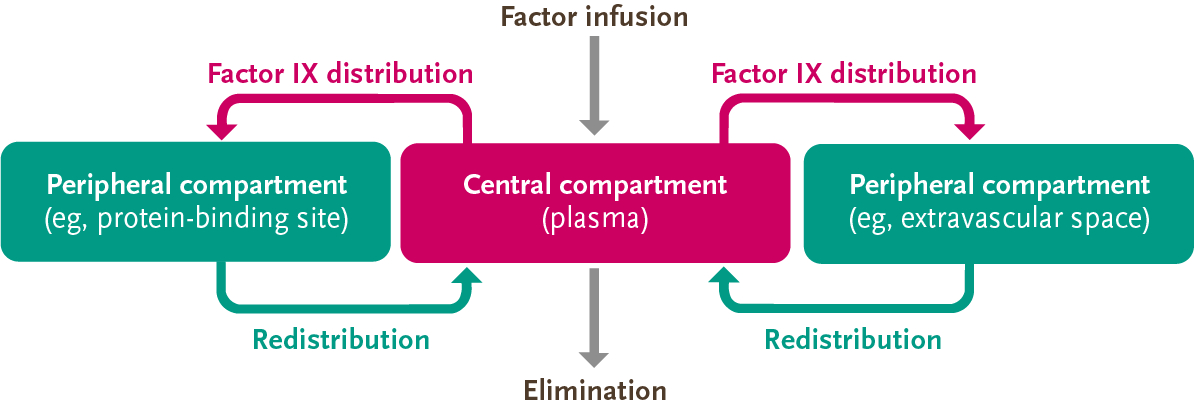
Adapted from Iorio, et al. Thromb Haemost. 2017.
- After factor IX enters the plasma, it rapidly distributes into peripheral compartments and eventually redistributes into the central compartment for elimination5
Because factor IX may reside in 3 different compartments, and trough only accounts for one compartment (plasma), trough may not account for all the factor IX activity in the body.5,13

Clinical studies have shown that factor IX spends more than half of its total time in the body outside the plasma3,5
Factor IX binds to type IV collagen, which may play a key role in coagulation15,18
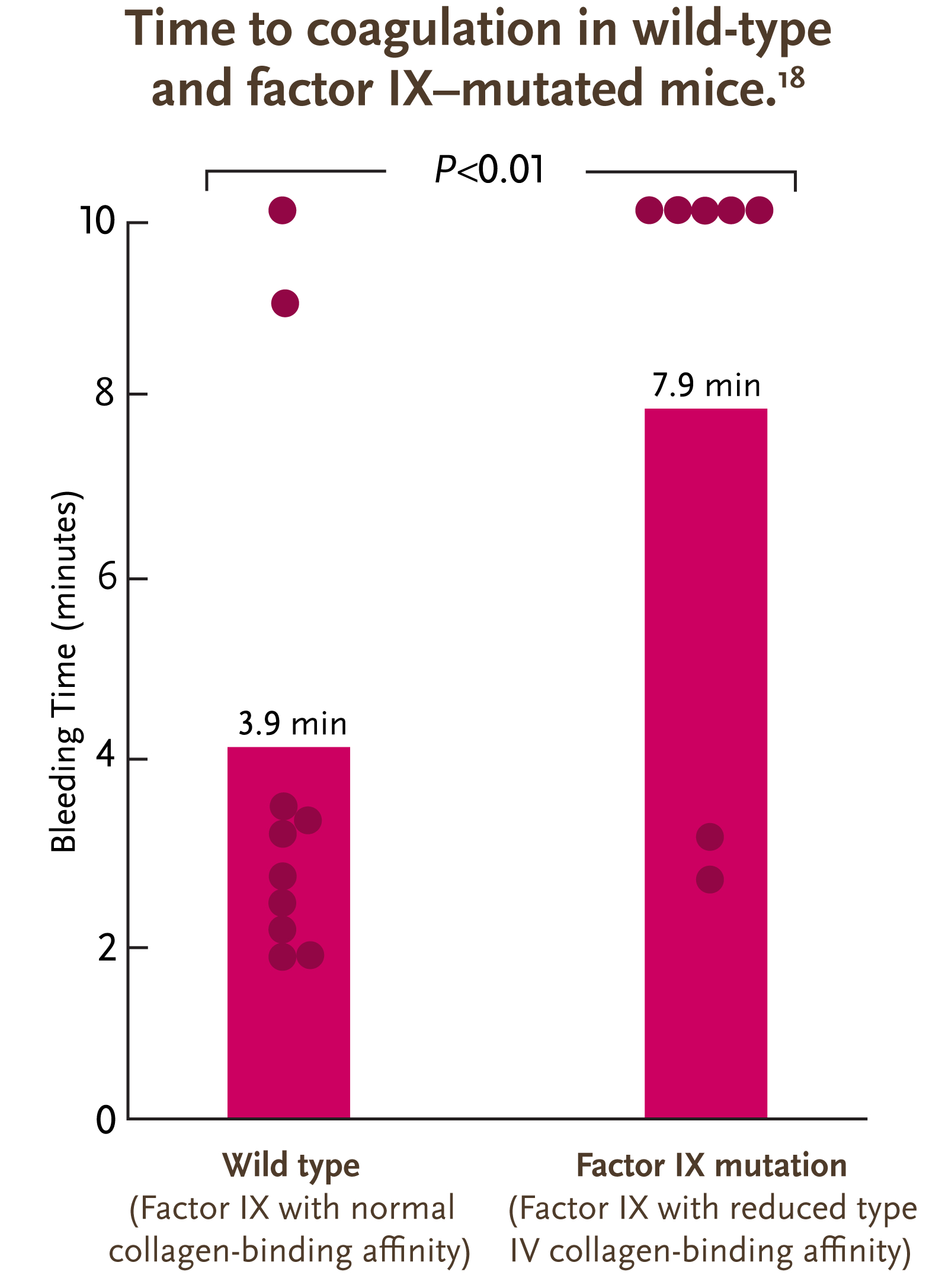
- A study in mice demonstrated that factor IX binds to type IV collagen in the extravascular space15
- A higher affinity to type IV collagen is associated with a significant reduction in bleeding time8
Adapted from Gui et al. J Thromb Haemost. 2009.
Abnormal hemostasis in a knock-in mouse carrying a variant of factor IX with impaired binding to collagen type IV
Gui, et al. J Thromb Haemost. 2009.
Gui et al conducted a study to determine the effect of reduced factor IX binding to type IV collagen.
The researchers infused mice with K5A-mutated factor IX, which drastically reduced the affinity of factor IX for type IV collagen.
This reduced affinity caused the plasma factor levels of the K5A-mutated mice to be significantly higher than the wild-type mice, as type IV collagen binding in the extravascular space was reduced. The study found that the mean bleeding time for K5A-mutated mice was significantly higher than that for wild-type mice.
The researchers concluded that factor IX molecules with reduced affinity for type IV collagen have altered hemostatic properties, indicating that type IV collagen binding in the extravascular space likely plays a key role in coagulation.
Additional research in humans is needed to confirm these findings.8
Purple dots represent the length of bleeding time from time of tail clip to bleeding cessation for each mouse. After 10 minutes, bleeding was terminated by firm pressure. Solid purple bars indicate mean bleeding times for the groups.
Due to the complexity of factor IX, evaluation of factor IX replacement therapies should look at multiple PK indicators5
Parameters
Peak
Maximum concentration observed after infusion13
Because other PK parameters may not provide the full picture of factor activity when considered alone, peak should be considered as part of a more comprehensive evaluation19
Volume of distribution
Drug distribution in the plasma and rest of the body2,13
Additional research is needed to understand the clinical implications of volume of distribution
AUC
Total concentration in the body in a given period of time13
AUC should be considered as part of a more comprehensive evaluation because other PK parameters may not provide the full picture of factor activity when considered alone19
Clearance
Amount of plasma free of drug in a given period of time5,13
The representation of clearance in the multicompartment model may explain why the half-life of factor IX is longer than that of factor VIII, even though factor IX clearance is higher5
Half-life
Time to half of initial concentration13
Because half-life may be affected by the extravascular distribution of factor IX, considering it alone may not provide the full picture of factor activity. Extravascular distribution may complicate the PK analysis of terminal half-life because the estimate will be different depending on the compartment model used5,13

Trough
Minimum concentration1,2
Evaluating trough alone during a PK assessment may not measure protection due to the complex behavior of infused factor IX20
The ISTH recommends that PK assessments include at least 6 parameters13
Trough levels, which measure factor in the plasma, may not always correlate with bleed rates1,2
Although clinical trials have not directly compared extended half-life factor IX replacement drugs, individual trials have shown21,22:

A broad range of trough levels
1% to 27%

A narrow range of bleed rates
1.04 to 1.40

A broad range of trough levels
1% to 27%

A narrow range of bleed rates
1.04 to 1.40
Multiple considerations may be needed to help evaluate the treatment and management of hemophilia B5,13
- Due to the distinct behavior and extravascular distribution of factor IX, there is a need to consider hemophilia B as a unique bleeding disorder5,13
- Trough alone may not provide a full picture of factor IX activity and should be one of multiple ways we measure bleed prevention in hemophilia B1,2,19,24,25
- A more complete PK assessment (including half-life, trough, peak, AUC, clearance, and volume of distribution) may provide a different way of thinking about bleed prevention in hemophilia B1,2,5,13,26
- A patient-centered approach that includes evaluation of bleed rates, joint bleed prevention, adherence, and quality of life should be considered when assessing your patients with hemophilia B25,27
INFOGRAPHIC: View factor IX differences
References
1. Dolan G, Benson G, Duffy A, et al. Blood Rev. 2018;32(1):52-60. 2. Mann DM, Stafford KA, Poon M-C, Matino D, Stafford DW. Haemophilia. 2021;27(3):332-339. 3. Castaman G, Matino D. Haematologica. 2019;104(9):1702-1709. 4. American Thrombosis and Hemostasis Network. ATHN research report. June 30, 2019. 5. Iorio A, Fischer K, Blanchette V, et al. Thromb Haemost. 2017;117(6):1023-1030. 6. Mazurkiewicz-Pisarek A, Plucienniczak G, Ciach T, Plucienniczak A. Acta Biochim Pol. 2016;63(1):11-16. 7. Orlova NA, Kovnir SV, Vorobiev II, Gabibov AG. Acta Naturae. 2012;4(2):62-73. 8. Feng D, Stafford KA, Broze GJ, Stafford DW. J Thromb Haemost. 2013;11(12):2176-2178. 9. Stern DM, Knitter G, Kisiel W, Nawroth PP. Br J Haematol. 1987;66(2):227-232. 10. Björkman S. Haemophilia. 2013;19(6):882-886. 11. Björkman S. Haemophilia. 2003;9(suppl 1):101-110. 12. Lenting PJ, Van Schooten CJM, Denis CV. J Thromb Haemost. 2007;5(7):1353-1360. 13. Ragni MV, Croteau SE, Morfini M, et al. J Thromb Haemost. 2018;16(7):1437-1441. 14. Morfini M. J Clin Med. 2017;6(3):35. 15. Gui T, Lin H-F, Jin D-Y, et al. Blood. 2002;100(1):153-158. 16. Björkman S. Edited by: Lee CA, Berntorp E, Hoots WK. Textbook of Hemophilia. Third Edition. Hoboken, NJ: John Wiley & Sons, Ltd. 2014:117-122. 17. Björkman S. Haemophilia. 2013;19(6):808-813. 18. Gui T, Reheman A, Ni H, et al. J Thromb Haemost. 2009;7(11):1843-1851. 19. Morfini M, Gherardini S. Ther Adv Hematol. 2018;9(6):149-162. 20. Stafford D. Thromb J. 2016;14(suppl 1):35. 21. Peters R, Harris T. Nat Rev Drug Discov. 2018;17(7):493-508. 22. Mahlangu JN. Ther Adv Hematol. 2018;9(11):335-346. 23. Kleiboer B, Nielsen B, Ma AD, Abajas Y, Monroe DM, Key NS. Haemophilia. 2020;26(1):e23-e25. 24. Collins PW, Young G, Knobe K, et al. Blood. 2014;124(26):3880-3886. 25. Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Haemophilia. 2013;19(1):e1-e47. 26. McNamara PJ, Leggas M. Drug Distribution. Edited by: Hacker M, Messer W, Bachmann K. Pharmacology: Principles and Practice. Burlington, MA: Elsevier Academic Press; 2009:113-127. 27. McLaughlin JM, Witkop ML, Lambing A, Anderson TL, Munn J, Tortella B. Haemophilia. 2014;20(4):506-512.
MAT-US-2007164-v7.0-09/2024