How Beyfortus® (nirsevimab-alip) 50 mg and 100 mg Injection real-world evidence measures up1
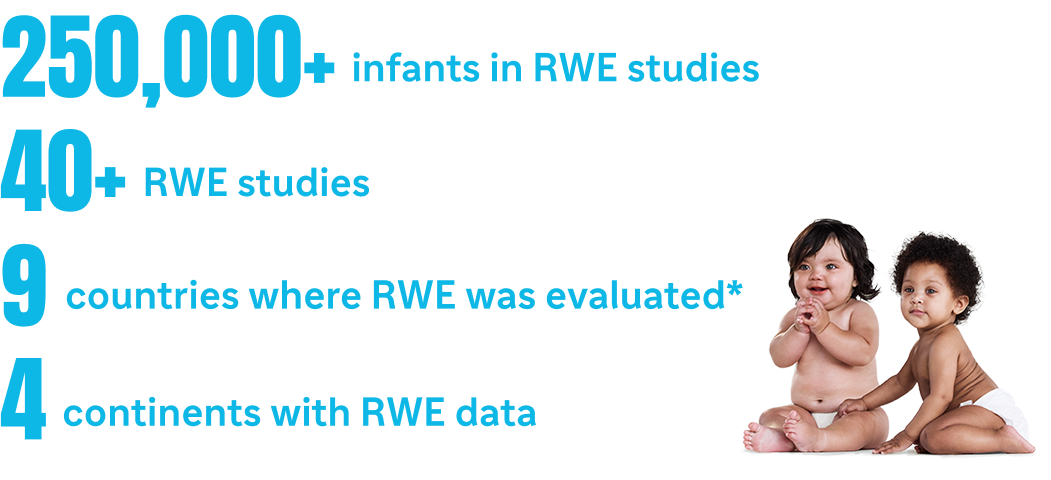
*In Andorra, Australia, Chile, France, Italy, Luxembourg, Portugal, Spain, and the United States as of March 05, 2025.1
Beyfortus Pivotal Trial Data
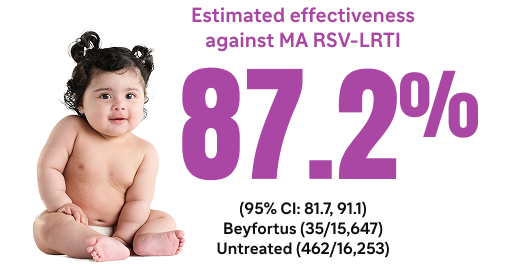
In 2 randomized, placebo-controlled pivotal trials, Beyfortus demonstrated efficacy against MA respiratory syncytial virus (RSV)-LRTI and RSV hospitalization1-5*
In Trial 04, Beyfortus exhibited a reduction in MA RSV hospitalization
compared to placebo: healthy term and late preterm infants (≥35 wGA)1-4
Trial 04 primary cohort†‡§

Trial 04 full study cohort‡

In Trial 03, Beyfortus exhibited a reduction in MA RSV-LRTI and RSV hospitalization compared to placebo: healthy preterm infants (≥29 to <35 wGA)2,5
Trial 03||¶

Trial 03 post hoc analysis#

Signs of LRTI involvement included rhonchi, rales, crackles, or wheezing and at least one sign of worsening clinical severity, including at least one of the following: increased respiratory rate, hypoxemia, acute hypoxic or ventilatory failure, new onset apnea, nasal flaring, retractions, grunting, or dehydration due to respiratory distress.2
CI, confidence interval; IM, intramuscular; MA RSV-LRTI, medically attended respiratory syncytial virus lower respiratory tract infection; RRR, relative risk reduction; RWE, real-world evidence; wGA weeks gestational age.
*Results of Trials 04 and 03 for infants entering their first RSV season.2
†Primary Cohort: 1,490 healthy term and late preterm infants (≥35 wGA) in Trial 04.2
‡During Trial 04, the COVID-19 pandemic interrupted trial enrollment. The efficacy analysis is based on the Primary Cohort, which included those participants enrolled prior to the pause due to COVID-19. Trial 04 continued monitoring the Primary Cohort and included an additional 1,522 subjects enrolled after the pause to comprise the full study cohort. The full study cohort included 3,012 infants randomized to receive Beyfortus or placebo in the post hoc analysis.4
§Efficacy for MA RSV-LRTI based on RRR against placebo, adjusted for age at randomization.2
||1,453 preterm infants (≥29 to <35 wGA) in Trial 03.2
¶Efficacy for MA RSV-LRTI based on RRR against placebo, adjusted for age at randomization and hemisphere.2
#860 infants from the full study cohort of 1,453 healthy preterm infants in Trial 03 were analyzed in a post hoc analysis. For infants in their first RSV season, the recommended dose is 50 mg for infants <5 kg or 100 mg for infants ≥5 kg via IM injection.1,2
**The Safety Population includes all infants who received the recommended dose of Beyfortus in Trials 04 and 03: Primary and Safety cohorts from Trial 04; infants who weighed <5 kg and who received the recommended dose of Beyfortus (single 50 mg IM dose) in Trial 03.2
Most common adverse reactions in Trial 04 and Trial 03 were rash (0.9%) and injection site reactions (0.3%).2**
Trial 04 and Trial 03 were pooled to evaluate the safety of Beyfortus (N=2,570) compared to placebo (N=1,284).2
• Adverse reactions were reported in 1.2% of infants who received Beyfortus; most (97%) of adverse reactions were mild to moderate in severity
Discover real-world data for Beyfortus

Beyfortus effectiveness against medically attended respiratory syncytial virus (RSV) events in infants (BEAR) is an observational, retrospective cohort study that assessed the effectiveness of Beyfortus at Kaiser Permanente Northern California (KPNC) during the 2023-2024 RSV season.6
Study design
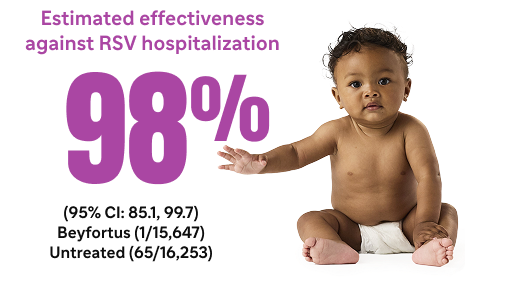
Beyfortus was associated with a reduction in MA RSV-LRTI and RSV hospitalization in healthy term US infants (aged <8 months and born at ≥37 wGA)6
This study included 31,900 healthy term infants aged <8 months (≥37 wGA), born on or after April 1, 2023, without a pre-defined high-risk condition during the 2023-2024 RSV season. Beyfortus was routinely administered beginning October 19, 2023, at KPNC.6*
- 15,647 infants received Beyfortus compared to 16,253 infants who were untreated
Co-primary endpoints6:
- First episode of PCR-confirmed RSV-LRTI during the RSV season†
- Healthcare utilization, as measured by the total number of medical encounters related to each RSV-LRTI episode‡
Post hoc analysis6:
- RSV hospitalization§
BEAR study results6
Co-Primary Endpoints6
Infants immunized with Beyfortus had fewer medical encounters and were less likely to be hospitalized vs untreated infants
Total RSV-LRTI medical encounters related to RSV-LRTI episodes

Post hoc analysis6
*There were 2 untreated infants who had 2 hospitalizations each during their RSV-LRTI episodes with a total of 67 hospitalizations across all 65 RSV-LRTI episodes.7
The findings in this analysis are subject to limitations6:
- Investigators were unable to assess how soon RSV-LRTI protection occurs after receiving Beyfortus because few cases occurred within 7 days
- Although KPNC performs a large amount of PCR testing, investigators did not quantify what proportion of infants with respiratory symptoms were tested, so testing biases among RSV-positive infants were possible
- As an observational study, unmeasured confounders that increase one’s risk of RSV (eg, lower socioeconomic status, having siblings, and/or daycare attendance) could affect results
- The analysis population of healthy term infants may not be generalizable to infants at higher risk of RSV-LRTI
- While KPNC has a large and diverse population, the study population may not be representative of other health systems’ populations
CI, confidence interval; ED, emergency department; MA RSV-LRTI, medically attended respiratory syncytial virus lower respiratory tract infection; PCR, polymerase chain reaction; RWE, real-world evidence; wGA, weeks’ gestational age.
*Eligible infants born on or after this date were offered the treatment before discharge, during urgent care visits, or at outpatient well-child appointments.
Eligible infants born prior to this date were contacted by KPNC as part of routine care for catch-up dosing in outpatient clinics.6
†An episode is defined as having at least 1 LRTI-associated encounter in any setting in the 7 days before and up to 10 days after a positive RSV PCR test.6
‡Each medical encounter was categorized as outpatient, emergency department, inpatient, or intensive care unit.6
§Episodes that included at least 1 hospitalization were considered a hospitalized RSV-LRTI.6
The analysis was a population-based, prospective, active surveillance study that assessed the effectiveness of Beyfortus within the New Vaccine Surveillance Network (NVSN) during the 2023-2024 RSV season.8
Study design
Beyfortus was associated with preventing MA RSV ARI and RSV hospitalization in US infants (aged <8 months8*
This study included infants <8 months as of October 1, 2023, or born after October 1, 2023, who had medically attended acute respiratory illness (ARI) between October 2023 and March 2024, who had verified Beyfortus status and medical records to assess underlying medical conditions, and were within the NVSN, which includes 7 sites.8,9†
- N=1,616 medically attended ARI (case patients receiving positive RSV test result, n=765, control patients receiving negative RSV test result, n=851)8‡
- Infants were considered Beyfortus recipients if they received Beyfortus ≥7 days before symptom onset9§
CDC real-world analysis results.8,9
Estimated effectiveness against MA RSV-ARI||
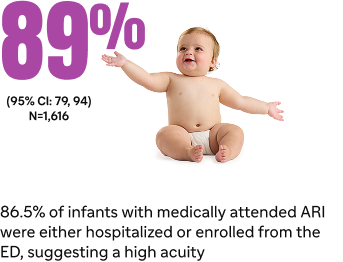
Estimated effectiveness against RSV hospitalization8¶#
Among RSV isolates, there were no variants in the Beyfortus binding site on the F protein that would alter susceptibility of RSV to Beyfortus8
The findings in this report are subject to limitations8:
- NVSN sites may not be nationally representative
- Timing of Beyfortus availability resulted in a relatively short interval from administration to respiratory illness onset
- Beyfortus effectiveness over the course of a full RSV season is expected to be somewhat lower than this estimate because of antibody decay
CDC, Centers for Disease Control and Prevention; MA RSV ARI, medically attended respiratory syncytial virus acute respiratory illness.
*Medically attended RSV was defined as any medically attended RSV-associated ARI and hospitalization was defined as RSV-associated hospitalization for ARI.9
†ARI is defined as one or more of the following signs or symptoms present for <14 days before enrollment encounter: apnea, cough, earache, fever, myalgia, nasal congestion, runny nose, sore throat, vomiting after coughing, shortness of breath (rapid or shallow breathing), wheezing, or apparent life-threatening event or brief resolved unexplained event.9
‡Ten out of 765 case patients who received Beyfortus were RSV positive compared with 126 out of 851 control patients who were negative for RSV.8,9
§Receipt of Beyfortus was ascertained through parent/guardian interviews, medical record abstraction, outpatient records, and state immunization information systems.8
‖Based on the median time from receipt of Beyfortus to symptom onset of 44 days (IQR=21-73 days).8
¶Based on the median time from receipt of Beyfortus to symptom onset of 41 days (IQR=18-68 days).9
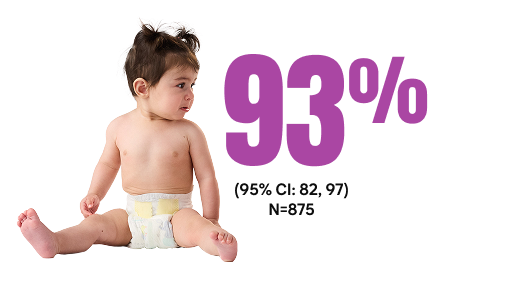
#Of 875 hospitalized patients, 531 (61%) were case patients with a positive RSV result and 344 (39%) were control patients with a negative RSV result. Six out of 531 case patients who received Beyfortus were RSV positive compared with 67 out of 344 control patients who were negative for RSV.8,9
.

HARMONIE assessed the reduction of RSV hospitalization with Beyfortus during the RSV season through 180 days.10,11
Study design
Beyfortus was associated with a reduction in RSV hospitalization in healthy term and preterm infants (aged ≤12 months and born at ≥29 wGA) through 180 days 10,11*
The phase 3b HARMONIE study was a pragmatic, open-label, randomized, parallel, 2-arm trial that studied Beyfortus vs no intervention in healthy term and preterm infants ≤12 months (≥29 wGA) entering their first RSV season in France, Germany, or the United Kingdom. The RSV season ended on February 28, 2023, in each country.11†
Primary endpoint: overall incidence of RSV hospitalization caused by confirmed RSV infection, through the RSV season10*†‡§
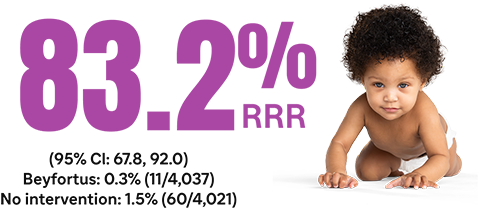
Secondary endpoint: overall incidence of RSV hospitalization through 180 days after randomization11†‡§||
50 mg IM dose if <5 kg weight, 100 mg IM dose if ≥5 kg weight

In most of the continental US, the typical RSV season lasts through 5 months, the same
length of time reported in the Beyfortus pivotal trials.2,12
The findings in this report are subject to limitations11:
- The follow-up does not include a second RSV season where most of the loss of efficacy would be expected to occur
- The possibility of bias exists because the study is unblinded; however, extensive efforts have been made to reduce the risk of bias, including the use of endpoints that are robust to the open-label nature of the study
- The HARMONIE study was conducted in the epidemiological setting typical for European countries, with a period of high RSV circulation followed by low circulation after February. The lower number of cases due to low viral circulation in the latter part of the follow-up makes it more difficult to definitively assess sustained protection
HARMONIE safety results11,13
Treatment-emergent adverse events (TEAEs) in safety analysis population: healthy infants aged ≤12 months, during 12 months following randomization.11,13
| Adverse events (AEs)¶ | Beyfortus, % (N=4,016) | No intervention, % (N=4,018) |
| Any TEAE | 80.0 | 79.4 |
| Immediate TEAE: ≤30 minutes post dosing/randomization | 0.7 | 0 |
| Any study treatment-related TEAE# | 2.5 | 0 |
| TEAE of special interest | 0.3 | <0.1 |
| Any serious TEAE | 6.5 | 5.5 |
| Any serious study treatment-related TEAE | <0.1 | 0 |
| Medically attended TEAE | 77.3 | 77.2 |
Most AEs in the 2 trial groups were grade 1 or 2 severity.11
IM, intramuscular.
*RSV hospitalization was defined as hospitalization for RSV-associated LRTI with hospital admission and an RSV-positive test result.11
†Infants could be born before or during the RSV season, which began on September 11, 2022 (week 37) in France; on October 9, 2022 (week 41) in Germany; and on September 4, 2022 (week 36) in the United Kingdom. The RSV season ended on February 28, 2023, in each country.11
‡Overall incidence of hospitalization for RSV-LRTI was defined as admission to the hospital on the basis of the treating physician’s decision and confirmation of RSV by means of a positive result of a test performed in accordance with routine practice, during the RSV season in France, Germany, and the United Kingdom.11
§Infants were randomized 1:1 to receive either a single IM injection of Beyfortus or standard of care (no intervention). For primary endpoint: N=8,058; Beyfortus n=4,037, no intervention n=4,021. For secondary endpoint: N=8,057; Beyfortus n=4,038, no intervention n=4,019. The characteristics of infants at randomization were similar in the 2 groups and 85.2% of infants were ≥37 wGA at birth.10,11
||The 2-sided 95% Cl for the efficacy is calculated by an exact method assuming a binomial distribution of the number of RSV-LRTI hospitalizations in the Beyfortus group conditional on the total number in both groups (described by Breslow and Day) accounting for the follow-up time after randomization.11,14
¶Assessment of AE severity was based on the AE severity grading scales adapted from FDA Guidance for Industry: Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials, September 2007.13
#The relationship between a TEAE and treatment was assessed as related or not related. A treatment-related TEAE was defined as a TEAE considered by the investigator as related to or with an unknown or missing relationship to treatment for participants who received Beyfortus on Day 1. An unknown or missing relationship between a TEAE and treatment for participants who received no intervention was considered not related (Medical Dictionary for Regulatory Activities Version 25.0).13
Beyfortus Pivotal Trial Data
In 2 randomized, placebo-controlled pivotal trials, Beyfortus demonstrated efficacy against MA respiratory syncytial virus (RSV)-LRTI and RSV hospitalization1-5*
In Trial 04, Beyfortus exhibited a reduction in MA RSV hospitalization
compared to placebo: healthy term and late preterm infants (≥35 wGA)1-4
Trial 04 primary cohort†‡§

Trial 04 full study cohort‡

In Trial 03, Beyfortus exhibited a reduction in MA RSV-LRTI and RSV hospitalization compared to placebo: healthy preterm infants (≥29 to <35 wGA)2,5
Trial 03||¶

Trial 03 post hoc analysis#

Signs of LRTI involvement included rhonchi, rales, crackles, or wheezing and at least one sign of worsening clinical severity, including at least one of the following: increased respiratory rate, hypoxemia, acute hypoxic or ventilatory failure, new onset apnea, nasal flaring, retractions, grunting, or dehydration due to respiratory distress.2
CI, confidence interval; IM, intramuscular; MA RSV-LRTI, medically attended respiratory syncytial virus lower respiratory tract infection; RRR, relative risk reduction; RWE, real-world evidence; wGA weeks gestational age.
*Results of Trials 04 and 03 for infants entering their first RSV season.2
†Primary Cohort: 1,490 healthy term and late preterm infants (≥35 wGA) in Trial 04.2
‡During Trial 04, the COVID-19 pandemic interrupted trial enrollment. The efficacy analysis is based on the Primary Cohort, which included those participants enrolled prior to the pause due to COVID-19. Trial 04 continued monitoring the Primary Cohort and included an additional 1,522 subjects enrolled after the pause to comprise the full study cohort. The full study cohort included 3,012 infants randomized to receive Beyfortus or placebo in the post hoc analysis.4
§Efficacy for MA RSV-LRTI based on RRR against placebo, adjusted for age at randomization.2
||1,453 preterm infants (≥29 to <35 wGA) in Trial 03.2
¶Efficacy for MA RSV-LRTI based on RRR against placebo, adjusted for age at randomization and hemisphere.2
#860 infants from the full study cohort of 1,453 healthy preterm infants in Trial 03 were analyzed in a post hoc analysis. For infants in their first RSV season, the recommended dose is 50 mg for infants <5 kg or 100 mg for infants ≥5 kg via IM injection.1,2
**The Safety Population includes all infants who received the recommended dose of Beyfortus in Trials 04 and 03: Primary and Safety cohorts from Trial 04; infants who weighed <5 kg and who received the recommended dose of Beyfortus (single 50 mg IM dose) in Trial 03.2
Most common adverse reactions in Trial 04 and Trial 03 were rash (0.9%) and injection site reactions (0.3%).2**
Trial 04 and Trial 03 were pooled to evaluate the safety of Beyfortus (N=2,570) compared to placebo (N=1,284).2
• Adverse reactions were reported in 1.2% of infants who received Beyfortus; most (97%) of adverse reactions were mild to moderate in severity
Discover real-world data for Beyfortus

Beyfortus effectiveness against medically attended respiratory syncytial virus (RSV) events in infants (BEAR) is an observational, retrospective cohort study that assessed the effectiveness of Beyfortus at Kaiser Permanente Northern California (KPNC) during the 2023-2024 RSV season.6
Study design
Beyfortus was associated with a reduction in MA RSV-LRTI and RSV hospitalization in healthy term US infants (aged <8 months and born at ≥37 wGA)6
This study included 31,900 healthy term infants aged <8 months (≥37 wGA), born on or after April 1, 2023, without a pre-defined high-risk condition during the 2023-2024 RSV season. Beyfortus was routinely administered beginning October 19, 2023, at KPNC.6*
- 15,647 infants received Beyfortus compared to 16,253 infants who were untreated
Co-primary endpoints6:
- First episode of PCR-confirmed RSV-LRTI during the RSV season†
- Healthcare utilization, as measured by the total number of medical encounters related to each RSV-LRTI episode‡
Post hoc analysis6:
- RSV hospitalization§
BEAR study results6
Co-Primary Endpoints6

Infants immunized with Beyfortus had fewer medical encounters and were less likely to be hospitalized vs untreated infants
Total RSV-LRTI medical encounters related to RSV-LRTI episodes

Post hoc analysis6

*There were 2 untreated infants who had 2 hospitalizations each during their RSV-LRTI episodes with a total of 67 hospitalizations across all 65 RSV-LRTI episodes.7
The findings in this analysis are subject to limitations6:
- Investigators were unable to assess how soon RSV-LRTI protection occurs after receiving Beyfortus because few cases occurred within 7 days
- Although KPNC performs a large amount of PCR testing, investigators did not quantify what proportion of infants with respiratory symptoms were tested, so testing biases among RSV-positive infants were possible
- As an observational study, unmeasured confounders that increase one’s risk of RSV (eg, lower socioeconomic status, having siblings, and/or daycare attendance) could affect results
- The analysis population of healthy term infants may not be generalizable to infants at higher risk of RSV-LRTI
- While KPNC has a large and diverse population, the study population may not be representative of other health systems’ populations
CI, confidence interval; ED, emergency department; MA RSV-LRTI, medically attended respiratory syncytial virus lower respiratory tract infection; PCR, polymerase chain reaction; RWE, real-world evidence; wGA, weeks’ gestational age.
*Eligible infants born on or after this date were offered the treatment before discharge, during urgent care visits, or at outpatient well-child appointments.
Eligible infants born prior to this date were contacted by KPNC as part of routine care for catch-up dosing in outpatient clinics.6
†An episode is defined as having at least 1 LRTI-associated encounter in any setting in the 7 days before and up to 10 days after a positive RSV PCR test.6
‡Each medical encounter was categorized as outpatient, emergency department, inpatient, or intensive care unit.6
§Episodes that included at least 1 hospitalization were considered a hospitalized RSV-LRTI.6
The analysis was a population-based, prospective, active surveillance study that assessed the effectiveness of Beyfortus within the New Vaccine Surveillance Network (NVSN) during the 2023-2024 RSV season.8
Study design
Beyfortus was associated with preventing MA RSV ARI and RSV hospitalization in US infants (aged <8 months8*
This study included infants <8 months as of October 1, 2023, or born after October 1, 2023, who had medically attended acute respiratory illness (ARI) between October 2023 and March 2024, who had verified Beyfortus status and medical records to assess underlying medical conditions, and were within the NVSN, which includes 7 sites.8,9†
- N=1,616 medically attended ARI (case patients receiving positive RSV test result, n=765, control patients receiving negative RSV test result, n=851)8‡
- Infants were considered Beyfortus recipients if they received Beyfortus ≥7 days before symptom onset9§
CDC real-world analysis results.8,9
Estimated effectiveness against MA RSV-ARI||

Estimated effectiveness against RSV hospitalization8¶#

Among RSV isolates, there were no variants in the Beyfortus binding site on the F protein that would alter susceptibility of RSV to Beyfortus8
The findings in this report are subject to limitations8:
- NVSN sites may not be nationally representative
- Timing of Beyfortus availability resulted in a relatively short interval from administration to respiratory illness onset
- Beyfortus effectiveness over the course of a full RSV season is expected to be somewhat lower than this estimate because of antibody decay
CDC, Centers for Disease Control and Prevention; MA RSV ARI, medically attended respiratory syncytial virus acute respiratory illness.
*Medically attended RSV was defined as any medically attended RSV-associated ARI and hospitalization was defined as RSV-associated hospitalization for ARI.9
†ARI is defined as one or more of the following signs or symptoms present for <14 days before enrollment encounter: apnea, cough, earache, fever, myalgia, nasal congestion, runny nose, sore throat, vomiting after coughing, shortness of breath (rapid or shallow breathing), wheezing, or apparent life-threatening event or brief resolved unexplained event.9
‡Ten out of 765 case patients who received Beyfortus were RSV positive compared with 126 out of 851 control patients who were negative for RSV.8,9
§Receipt of Beyfortus was ascertained through parent/guardian interviews, medical record abstraction, outpatient records, and state immunization information systems.8
‖Based on the median time from receipt of Beyfortus to symptom onset of 44 days (IQR=21-73 days).8
¶Based on the median time from receipt of Beyfortus to symptom onset of 41 days (IQR=18-68 days).9
#Of 875 hospitalized patients, 531 (61%) were case patients with a positive RSV result and 344 (39%) were control patients with a negative RSV result. Six out of 531 case patients who received Beyfortus were RSV positive compared with 67 out of 344 control patients who were negative for RSV.8,9
.

HARMONIE assessed the reduction of RSV hospitalization with Beyfortus during the RSV season through 180 days.10,11
Study design
Beyfortus was associated with a reduction in RSV hospitalization in healthy term and preterm infants (aged ≤12 months and born at ≥29 wGA) through 180 days 10,11*
The phase 3b HARMONIE study was a pragmatic, open-label, randomized, parallel, 2-arm trial that studied Beyfortus vs no intervention in healthy term and preterm infants ≤12 months (≥29 wGA) entering their first RSV season in France, Germany, or the United Kingdom. The RSV season ended on February 28, 2023, in each country.11†
Primary endpoint: overall incidence of RSV hospitalization caused by confirmed RSV infection, through the RSV season10*†‡§

Secondary endpoint: overall incidence of RSV hospitalization through 180 days after randomization11†‡§||
50 mg IM dose if <5 kg weight, 100 mg IM dose if ≥5 kg weight

In most of the continental US, the typical RSV season lasts through 5 months, the same
length of time reported in the Beyfortus pivotal trials.2,12
The findings in this report are subject to limitations11:
- The follow-up does not include a second RSV season where most of the loss of efficacy would be expected to occur
- The possibility of bias exists because the study is unblinded; however, extensive efforts have been made to reduce the risk of bias, including the use of endpoints that are robust to the open-label nature of the study
- The HARMONIE study was conducted in the epidemiological setting typical for European countries, with a period of high RSV circulation followed by low circulation after February. The lower number of cases due to low viral circulation in the latter part of the follow-up makes it more difficult to definitively assess sustained protection
HARMONIE safety results11,13
Treatment-emergent adverse events (TEAEs) in safety analysis population: healthy infants aged ≤12 months, during 12 months following randomization.11,13
| Adverse events (AEs)¶ | Beyfortus, % (N=4,016) | No intervention, % (N=4,018) |
| Any TEAE | 80.0 | 79.4 |
| Immediate TEAE: ≤30 minutes post dosing/randomization | 0.7 | 0 |
| Any study treatment-related TEAE# | 2.5 | 0 |
| TEAE of special interest | 0.3 | <0.1 |
| Any serious TEAE | 6.5 | 5.5 |
| Any serious study treatment-related TEAE | <0.1 | 0 |
| Medically attended TEAE | 77.3 | 77.2 |
Most AEs in the 2 trial groups were grade 1 or 2 severity.11
IM, intramuscular.
*RSV hospitalization was defined as hospitalization for RSV-associated LRTI with hospital admission and an RSV-positive test result.11
†Infants could be born before or during the RSV season, which began on September 11, 2022 (week 37) in France; on October 9, 2022 (week 41) in Germany; and on September 4, 2022 (week 36) in the United Kingdom. The RSV season ended on February 28, 2023, in each country.11
‡Overall incidence of hospitalization for RSV-LRTI was defined as admission to the hospital on the basis of the treating physician’s decision and confirmation of RSV by means of a positive result of a test performed in accordance with routine practice, during the RSV season in France, Germany, and the United Kingdom.11
§Infants were randomized 1:1 to receive either a single IM injection of Beyfortus or standard of care (no intervention). For primary endpoint: N=8,058; Beyfortus n=4,037, no intervention n=4,021. For secondary endpoint: N=8,057; Beyfortus n=4,038, no intervention n=4,019. The characteristics of infants at randomization were similar in the 2 groups and 85.2% of infants were ≥37 wGA at birth.10,11
||The 2-sided 95% Cl for the efficacy is calculated by an exact method assuming a binomial distribution of the number of RSV-LRTI hospitalizations in the Beyfortus group conditional on the total number in both groups (described by Breslow and Day) accounting for the follow-up time after randomization.11,14
¶Assessment of AE severity was based on the AE severity grading scales adapted from FDA Guidance for Industry: Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials, September 2007.13
#The relationship between a TEAE and treatment was assessed as related or not related. A treatment-related TEAE was defined as a TEAE considered by the investigator as related to or with an unknown or missing relationship to treatment for participants who received Beyfortus on Day 1. An unknown or missing relationship between a TEAE and treatment for participants who received no intervention was considered not related (Medical Dictionary for Regulatory Activities Version 25.0).13
Important Safety Information
References: 1. Data on File. Sanofi. 2. Beyfortus (nirsevimab-alip). Prescribing Information. Sanofi. 3. Muller WJ, Madhi SA, Nuñez BS, et al; MELODY Study Group. Nirsevimab for prevention of RSV in term and late-preterm infants. N Engl J Med. 2023;388(16):1533-1534. 4. Muller WJ, Madhi SA, Nuñez BS, et al; MELODY Study Group. Nirsevimab for prevention of RSV in term and late-preterm infants. N Engl J Med. 2023;388(16)(suppl):1533-1534. 5. Griffin MP, Yuan Y, Takas T, et al. Single-dose nirsevimab for prevention of RSV in preterm infants. N Engl J Med. 2020;383(5):415-425. 6. Hsiao A, Hansen J, Fireman B, et al. Effectiveness of nirsevimab against RSV and RSV-related events in infants. Pediatrics. 2025;156(2):e2024069510. 7. Hsiao A, Hansen J, Fireman B, et al. Effectiveness of nirsevimab against RSV and RSV-related events in infants. Pediatrics. 2025;156(2)(suppl):e2024069510. 8. Moline HL, Toepfer AP, Tannis A, et al; New Vaccine Surveillance Network Collaborators. Respiratory syncytial virus disease burden and nirsevimab effectiveness in young children from 2023-2024. JAMA Pediatr. 2025;179(2):179-187. 9. Moline HL, Toepfer AP, Tannis A, et al; New Vaccine Surveillance Network Collaborators. Respiratory syncytial virus disease burden and nirsevimab effectiveness in young children from 2023-2024. JAMA Pediatr. 2025;179(2)(suppl):179-187. 10. Drysdale SB, Cathie K, Flamein F, et al; HARMONIE Study Group. Nirsevimab for prevention of hospitalizations due to RSV in infants. N Engl J Med. 2023;389(26):2425-2435. 11. Munro APS, Drysdale SB, Cathie K, et al; HARMONIE Study Group. 180-day efficacy of nirsevimab against hospitalisation for respiratory syncytial virus lower respiratory tract infections in infants (HARMONIE): a randomised, controlled, phase 3b trial. Lancet Child Adolesc Health. 2025;9(6):404-412. 12. Obando-Pacheco P, Justicia-Grande AJ, Rivero-Calle I, et al. Respiratory syncytial virus seasonality: a global overview. J Infect Dis. 2018;217(9):1356-1364. 13. Munro APS, Drysdale SB, Cathie K, et al; HARMONIE Study Group. 180-day efficacy of nirsevimab against hospitalisation for respiratory syncytial virus lower respiratory tract infections in infants (HARMONIE): a randomised, controlled, phase 3b trial. Lancet Child Adolesc Health. 2025;9(6)(suppl):404-412. 14. Drysdale SB, Cathie K, Flamein F, et al; HARMONIE Study Group. Nirsevimab for prevention of hospitalizations due to RSV in infants. N Engl J Med. 2023;389(26)(protocol):2425-2435.