Beyfortus® (nirsevimab-alip) is a single dose timed to the respiratory syncytial virus (RSV) season for the vast majority of infants1
Beyfortus is indicated for the prevention of RSV lower respiratory tract disease in1:
- Neonates and infants born during or entering their first RSV season.
- Children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season.
Administration schedule
The ideal timing for Beyfortus dosing is just before or near the start of the first RSV season or from birth for infants born shortly before or during the RSV season.1,2
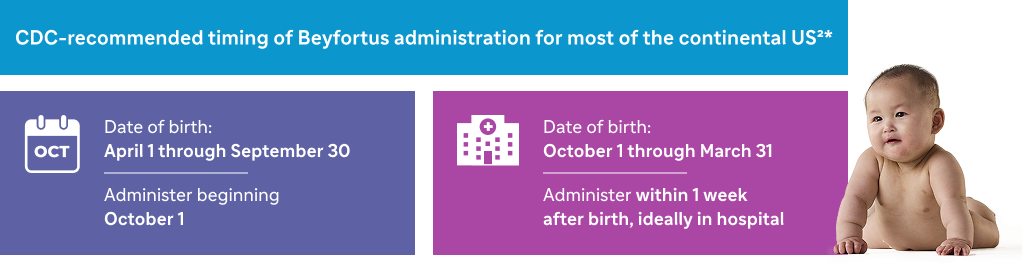
First RSV season
Neonates and infants born during or entering their first RSV season1
CDC recommends Beyfortus for infants aged <8 months between October 1 and March 312*
RSV is seasonal, with infections peaking at certain times throughout the year. A typical RSV season runs roughly from fall through spring, with some exceptions, such as in Florida and Hawaii, where the RSV season may start earlier. In most of the continental US, RSV typically peaks between December and January.1-4
*Timing of administration for RSV immunization may differ in certain areas.2
For full recommendations, which include important guidance, visit the CDC website.
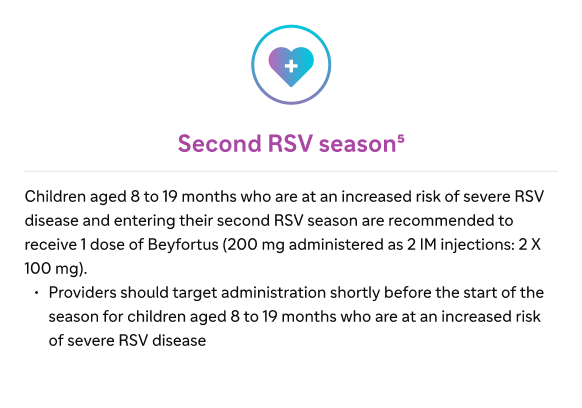
Second RSV season
Children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season1
Infants and children aged 8 to 19 months at increased risk for severe RSV disease who are recommended to receive Beyfortus when entering their second RSV season include5:
- Children with chronic lung disease of prematurity who required medical support (chronic corticosteroid therapy, diuretic therapy, or supplemental oxygen) any time during the 6-month period before the start of the second RSV season
- Severely immunocompromised children
- American Indian or Alaska Native children
- Children with cystic fibrosis who have either:
- manifestations of severe lung disease (previous hospitalization for pulmonary exacerbation in the first year of life or abnormalities on chest imaging that persist when stable), or
- weight-for-length <10th percentile
Special circumstances
For children undergoing cardiac surgery with cardiopulmonary bypass, an additional dose of Beyfortus is recommended as soon as the child is stable after surgery. Please consult the Prescribing Information for complete information on dosing in these circumstances.1

Weight-based dosing1

The recommended dosage of Beyfortus for neonates and infants born during or entering their first RSV season is based on body weight and is administered as a single IM injection.
For children up to 24 months of age, regardless of body weight, who remain at increased risk for severe RSV, the recommended dosage of Beyfortus is a single 200 mg dose administered as 2 IM injections (2 x 100 mg).
Recommended dosage of Beyfortus for their first RSV season1
| Body Weight at the Time of Dosing | Recommended Dosage |
| <5 kg | 50 mg by IM injection |
| ≥5 kg | 100 mg by IM injection |
Administration and storage1


Beyfortus can be concomitantly administered with childhood vaccines.1*
*There is limited experience of Beyfortus coadministration with vaccines. In clinical trials, when Beyfortus was given with routine childhood vaccines, the safety and reactogenicity profile of the coadministered regimen was similar to the childhood vaccines given alone. Beyfortus should not be mixed with any vaccine in the same syringe or vial. When administered concomitantly with injectable vaccines, they should be given with separate syringes and at different injection sites.
Regional considerations
ACIP regional administration guidelines5
Because the timing of the onset, peak, and decline of RSV activity might vary geographically, providers can adjust administration schedules based on local epidemiology.


Providers in these jurisdictions should consult state, local, or territorial guidance on timing of Beyfortus administration.
AAP and ACIP recommended5,7
Considerations regarding maternal vaccination8,9*
Beyfortus is recommended for infants aged <8 months born during or entering their first RSV season:
- Whose mother did not receive the RSV maternal vaccine
- Whose mother's receipt of the RSV maternal vaccine is unknown
- Who were born <14 days after maternal vaccination
- Beyfortus may be considered for infants whose mother received the RSV maternal vaccine, if, based on the clinical judgment of the healthcare provider, the potential incremental benefit of administration is warranted
- Current recommendations for 2024-2025 are for a single lifetime dose of the RSV maternal vaccine during pregnancy and that Beyfortus should be used for infants born to mothers who received the RSV vaccine during a previous pregnancy
Beyfortus is the first RSV prevention therapy approved for all infants in the US through their first season and the only infant RSV long-acting antibody indicated for a second RSV season1,10

*Beyfortus is not needed for most infants aged <8 months whose mother received the RSV maternal vaccine >14 days before birth.10
For more information about the dosing and administration of Beyfortus, please contact our representatives at VaccineShop
AAP, American Academy of Pediatrics; ACIP, Advisory Committee on Immunization Practices; CDC, Centers for Disease Control and Prevention; IM, intramuscular; US, United States; VFC, Vaccines for Children.
Important Safety Information
References: 1. Beyfortus (nirsevimab-alip). Prescribing Information. Sanofi. 2. Immunizations to protect infants. Centers for Disease Control and Prevention. Updated August 30, 2024. Accessed June 10, 2025. https://www.cdc.gov/rsv/vaccines/protect-infants.html 3. Surveillance of RSV. Centers for Disease Control and Prevention. Updated July 8, 2025. Accessed July 16, 2025. https://www.cdc.gov/rsv/php/surveillance/index.html 4. Rose EB, Wheatley A, Langley G, et al. Respiratory syncytial virus seasonality—United States, 2014-2017. MMWR Morb Mortal Wkly Rep. 2018;67(2):71-76. 5. Jones JM, Fleming-Dutra KE, Prill MM, et al. Use of nirsevimab for the prevention of respiratory syncytial virus disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(34):920-925. 6. Obando-Pacheco P, Justicia-Grande AJ, Rivero-Calle I, et al. Respiratory syncytial virus seasonality: a global overview. J Infect Dis. 2018;217(9):1356-1364. 7. AAP recommendations for the prevention of RSV disease in infants and children. American Academy of Pediatrics. Updated July 8, 2025. Accessed July 22, 2025. https://publications.aap.org/redbook/resources/25379/AAP-Recommendations-for-the-Prevention-of-RSV 8. Fleming-Dutra KE, Jones JM, Roper LE, et al. Use of the Pfizer respiratory syncytial virus vaccine during pregnancy for the prevention of respiratory syncytial virus–associated lower respiratory tract disease in infants: recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Mortal Wkly Rep. 2023;72(41):1115-1122. 9. RSV vaccine guidance for pregnant women. Centers for Disease Control and Prevention. Updated August 30, 2024. Accessed April 4, 2025. https://www.cdc.gov/rsv/hcp/vaccine-clinical-guidance/pregnant-people.html 10. RSV immunization guidance for infants and young children. Centers for Disease Control and Prevention. Updated August 30, 2024. Accessed May 5, 2025. https://www.cdc.gov/rsv/hcp/vaccine-clinical-guidance/infants-young-children.html

