- Article
- Source: Campus Sanofi
- 21 Mar 2025
Role of type 2 inflammation

Type 2 Inflammation may increase the risk of exacerbations and lung function impairment in COPD6,7
COPD is characterised by mucus production, airway obstruction, and coughing8
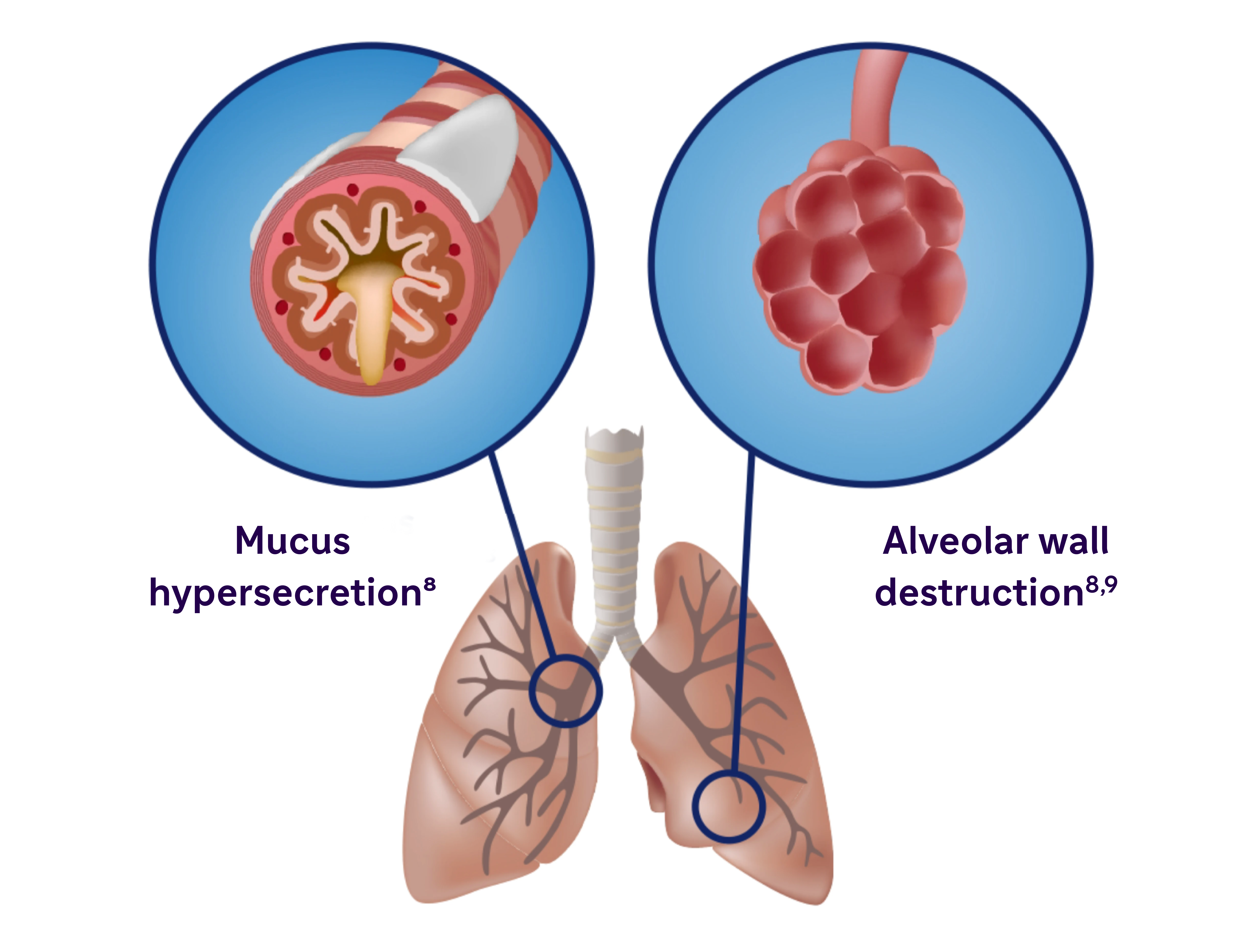
Inflammation manifests systemically and locally
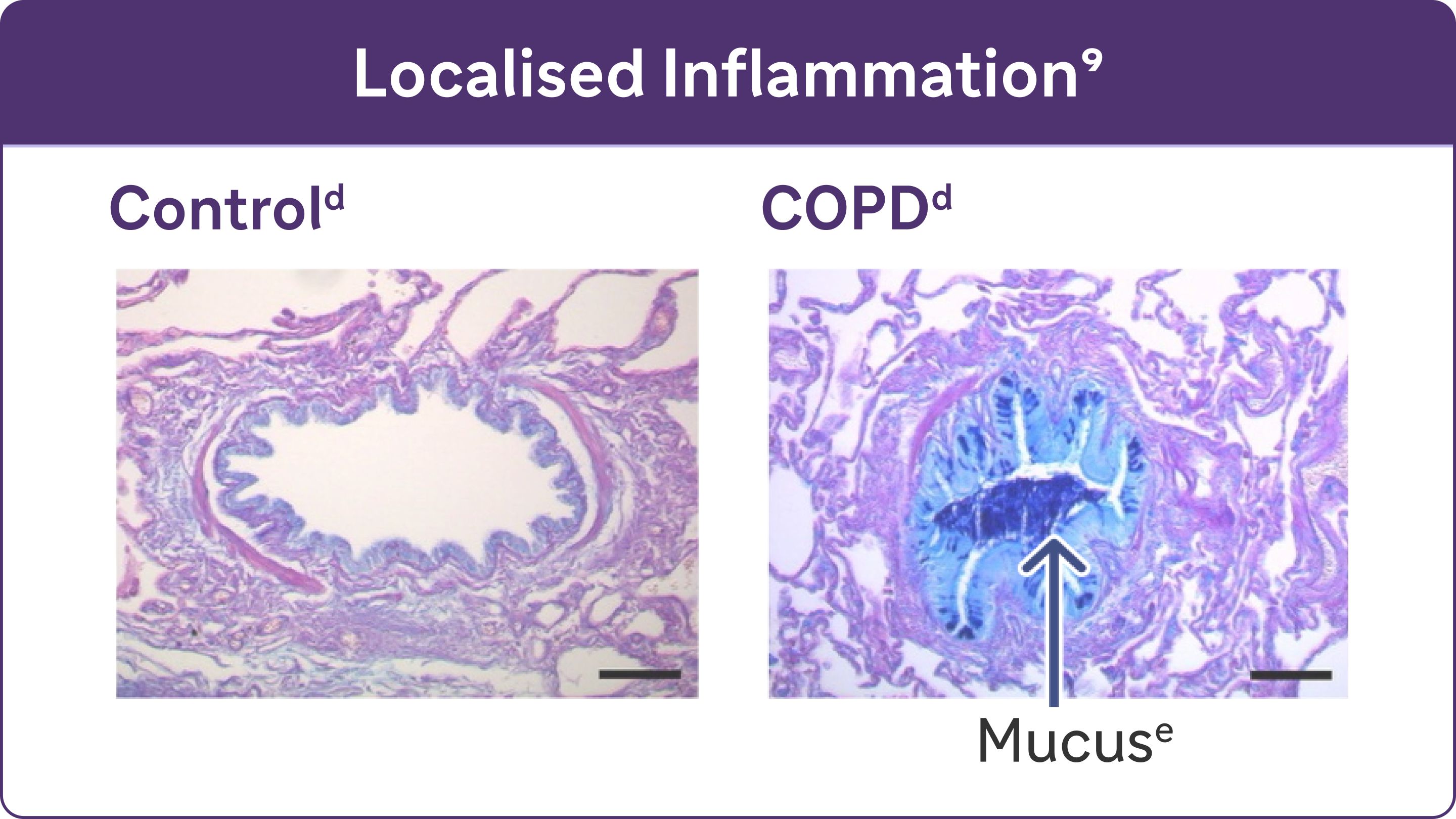
- Chronic inflammation causes structural changes, including narrowing of the airways and decreased lung elasticity8
- Studies have identified a relationship between inflammation and mucus hypersecretion in respiratory conditions such as COPD9

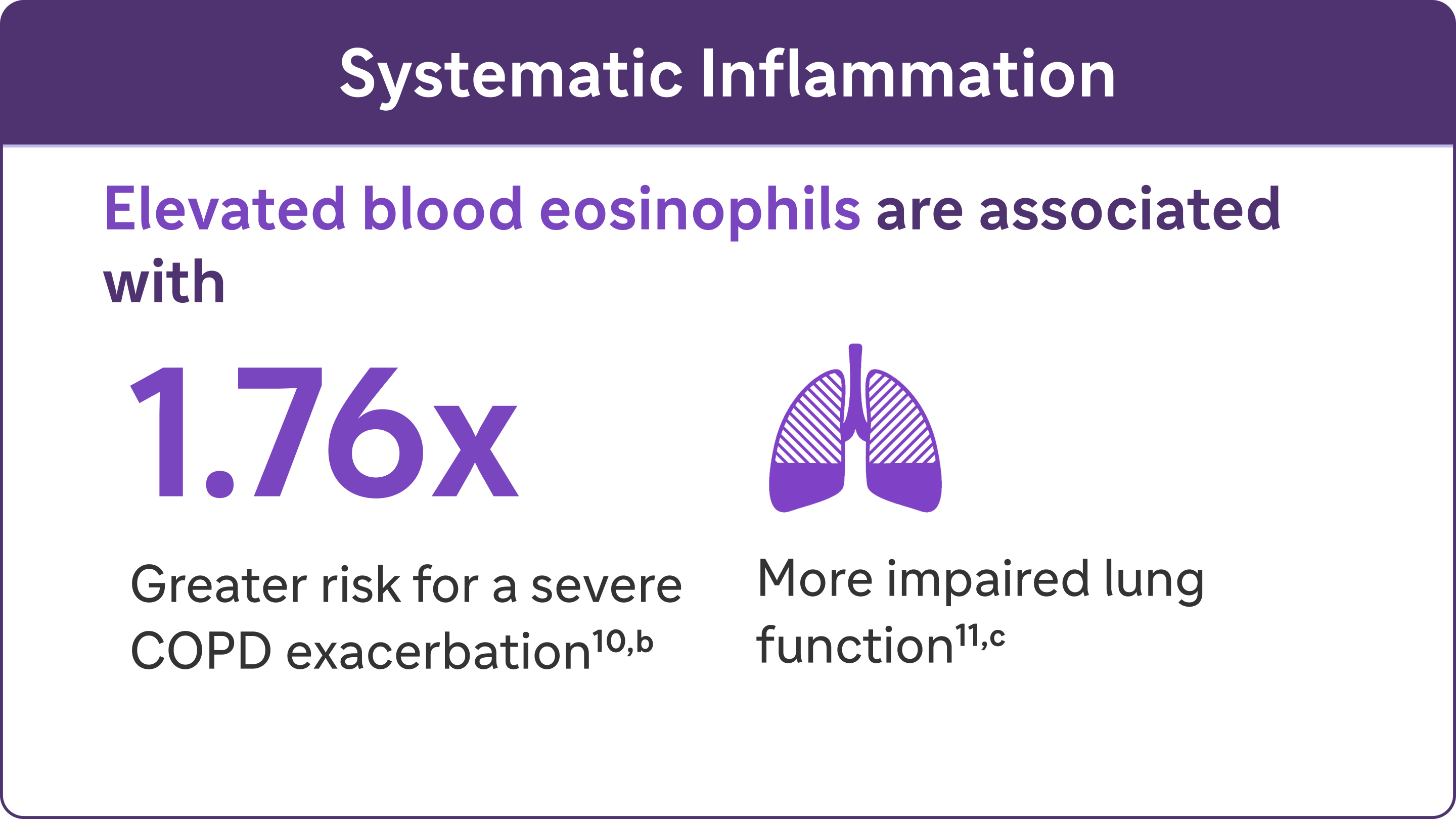
Look for elevated blood eosinophils - A Biomarker of Type 2 Inflammation in COPD8
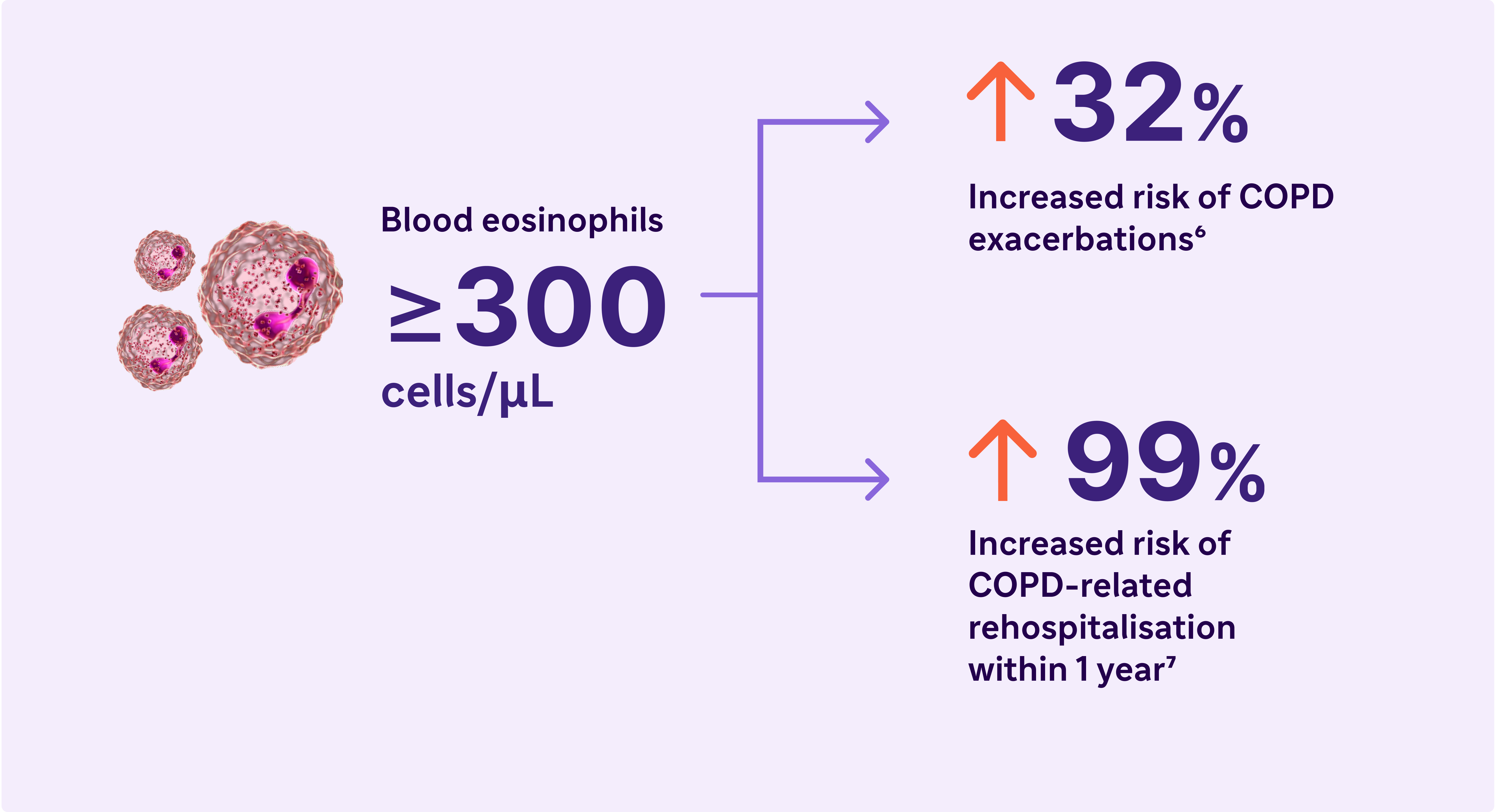
Elevated eosinophils in COPD are a treatable trait and marker of type 2 inflammation11
The 2024 GOLD Report recognises elevated blood EOS as a clinically used biomarker in identifying COPD with type 2 inflammation8
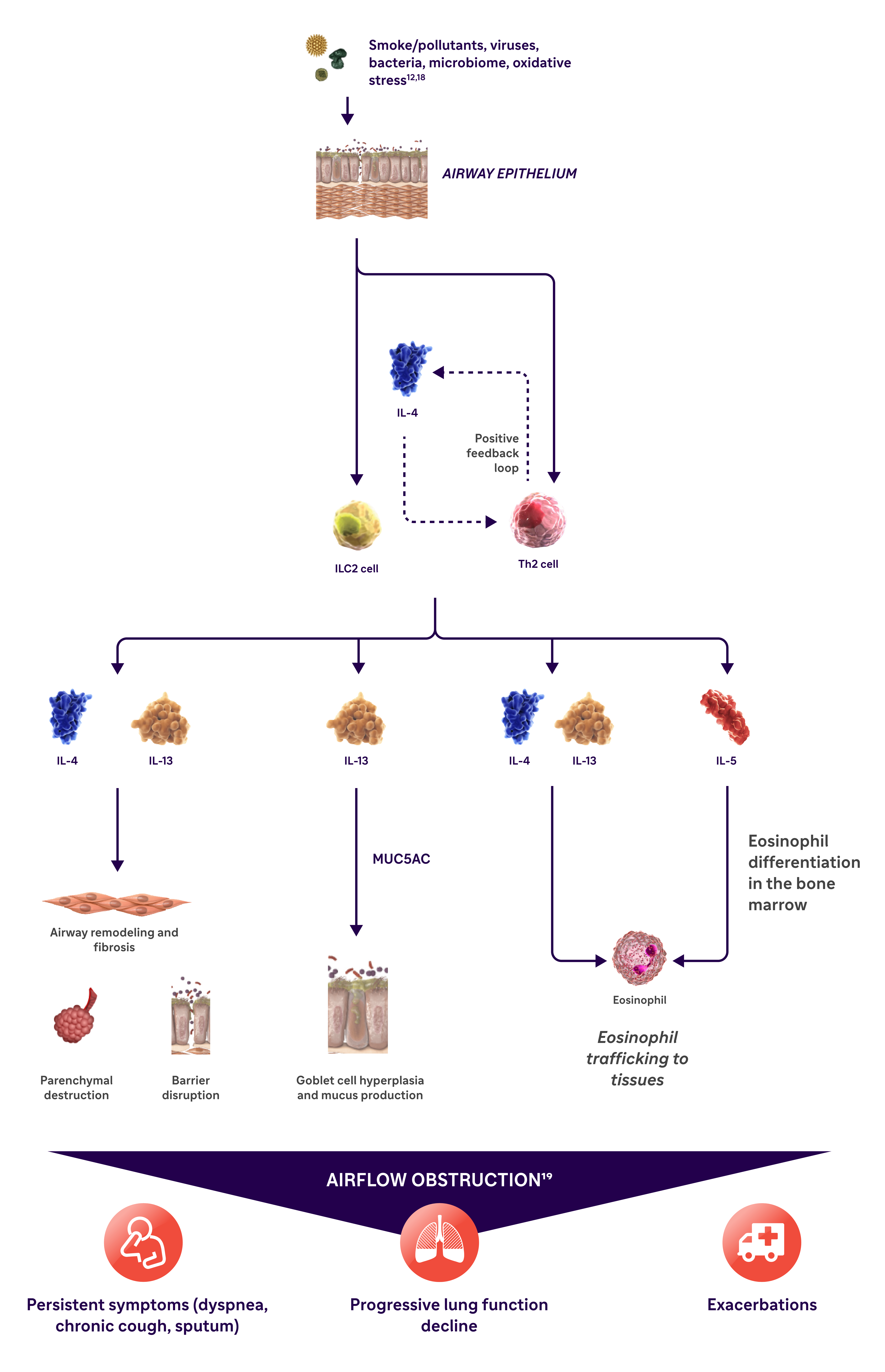
Type 2 inflammation in COPD can involve multiple pathways, cytokines, and inflammatory cells12
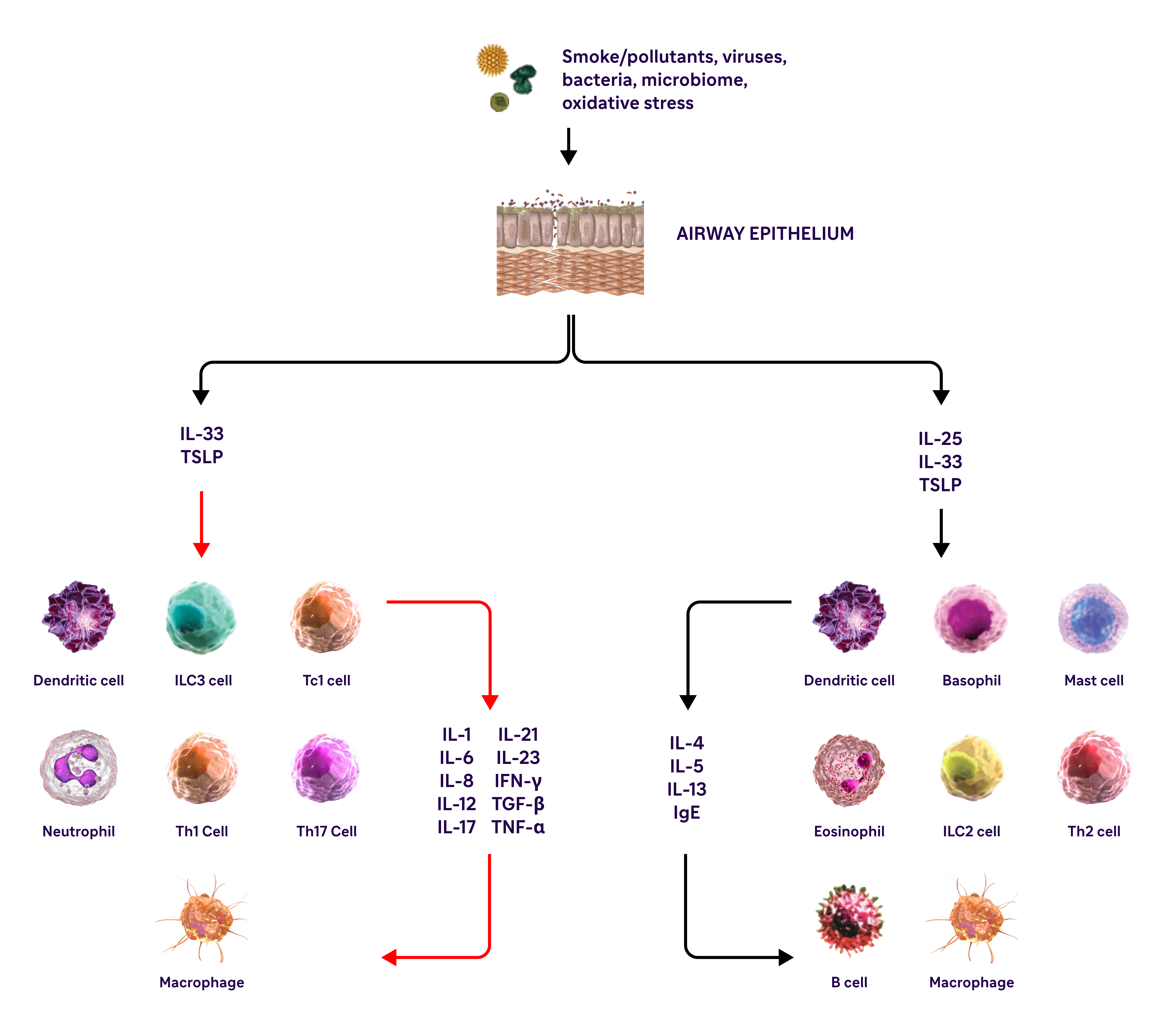
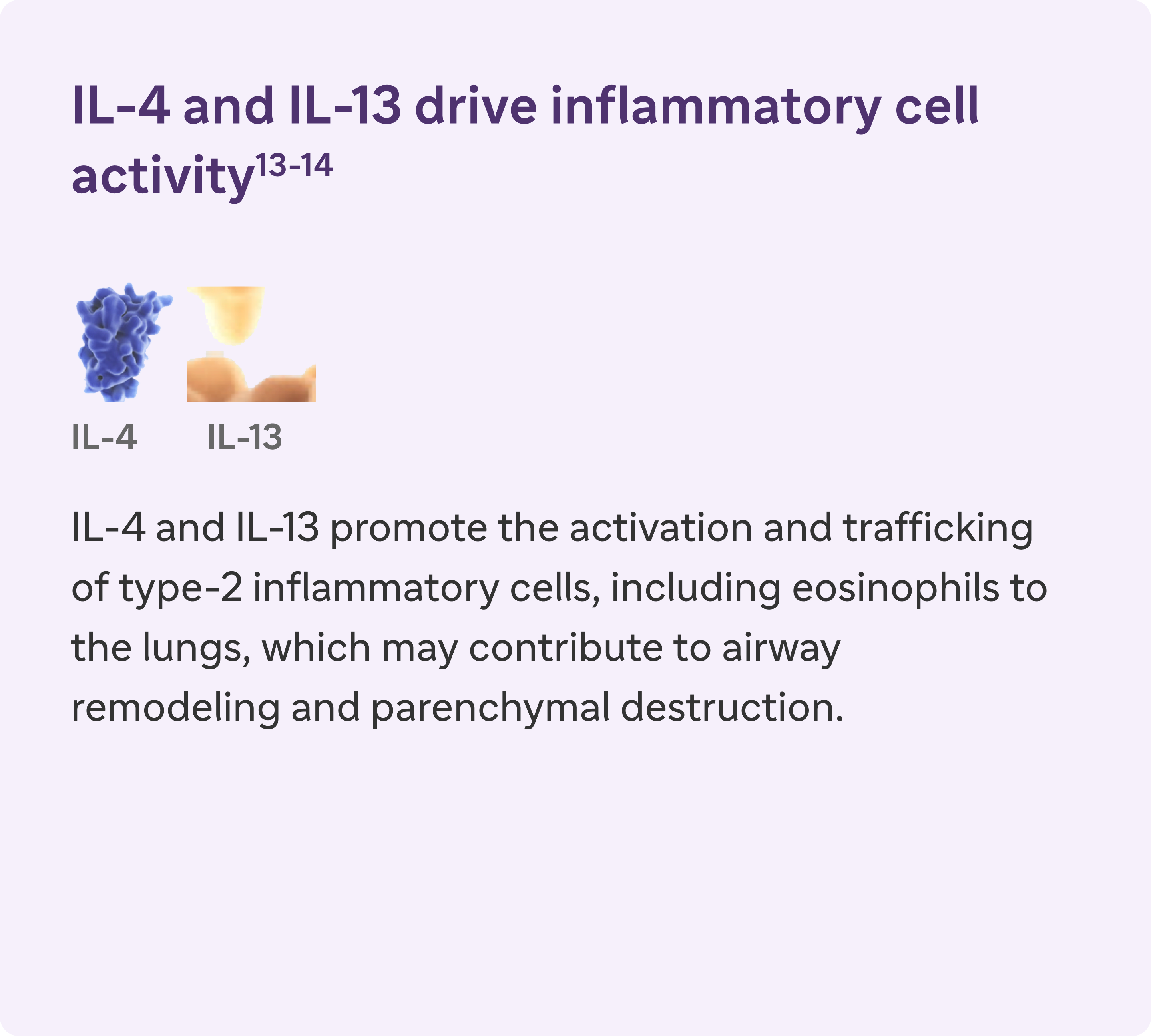
IL-4, IL-13, and IL-5 are type 2 cytokines involved in COPD


aBased on findings from 5 studies in COPD patients without asthma. Eosinophil levels used to define type 2 inflammation ranged from ≥300 cells/μL to ≥340 cells/μL (blood), ≥2% in induced sputum or 3% in peripheral blood. Percentages of patients with type 2 inflammation ranged from 12.3% to ~40%6,7
bA severe exacerbation was defined as a hospitalisation due to COPD. Exacerbations had to be a minimum of 4 weeks apart to be considered separate exacerbations.10
cIn a cohort of patients with EOS >200 cells/μL.9
dReproduced with permission of the American Thoracic Society. Fritzsching B et al. Am J Respir Crit Care Med. 2015;191(8):902-9139
eAlcian blue PAS staining of mucus in airway epithelial cells.9
fResults from an observational study of 1553 patients with GOLD spirometry grade 2-4 COPD (postbronchodilator FEV1/FVC ratio <0.7, with FEV, >80% predicted)6
gResults from a 1-year observational study of 479 patients with COPD, 173 of whom had blood eosinophil levels ≥200 cells/μL and/or ≥2% of the total white blood cell count.7
COPD, chronic obstructive pulmonary disease; EOS, eosinophils; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; IFN-y, interferon-gamma; ILC2, innate lymphoid type-2 cells; MUC5AC, mucin 5AC; PAS, periodic acid-Schiff; TNF-a, tumor necrosis factor alpha; TSLP, thymic stromal lymphopoietin.
References
- Oshagbemi OA, Franssen FME, van Kraaij S, et al. Blood eosinophil counts, withdrawal of inhaled corticosteroids and risk of COPD exacerbations and mortality in the clinical practice research datalink (CPRD). COPD. 2019;16:152-159.
- Casanova C, Celli BR, de-Torres JP, et al. Prevalence of persistent blood eosinophilia: relation to outcomes in patients with COPD. Eur Respir J. 2017;50:1701162.
- Singh D, Kolsum U, Brightling CE, et al; ECLIPSE investigators. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44:1697-1700.
- Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Grit Care Med. 2011;184:662-671.
- Oshagbemi OA, Burden AM, Braeken DCW, et al. Stability of blood eosinophils in patients with chronic obstructive pulmonary disease and in control subjects, and the impact of sex, age, smoking, and baseline counts. Am J Respir Crit Care Med. 2017;195:1402-1404.
- Yun JH, Lamb A, Chase R, et al; COPDGene and ECLIPSE Investigators. Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin lmmunol. 2018;141:2037-2047.
- Belanger M, Couillard S, Courteau J, et al. Eosinophil counts in first COPD hospitalizations: a comparison of health service utilization. Int J Chron Obstruct Pulmon Dis. 2018;13:3045-3054.
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2024 report). Available at https://goldcopd.org/2024-gold-report/ Accessed 23/07/2024.
- Fritzsching B, Zhou-Suckow Z, Trojanek JB, et al. Hypoxic epithelial necrosis triggers neutrophilic inflammation via IL-1 receptor signaling in cystic fibrosis lung disease. Am J Respir Crit Care Med. 2015;191:902-913.
- Vedel-Krogh S, et al. Blood Eosinophils and Exacerbations in Chronic Obstructive Pulmonary Disease. The Copenhagen General Population Study. Am J Respir Crit Care Med. 2016;193(9):965-74.
- Agustí A, Rapsomaniki E, Beasley R, et al. Treatable traits in the NOVELTY study. Respirology. 2022;27:929-40.
- Yousuf A, Ibrahim W, Greening NJ, et al. T2 Biologics for Chronic Obstructive Pulmonary Disease J Allergy Clin Immunol Pract. 2019;7:1405-16.
- Gandhi NA, Brandy BL, Graham NHM, et al. Targeting key proximal drivers of type 2 inflammation in disease. Nature Reviews Drug Discovery. 2016;15:35-50.
- Saatian B, Rezaee F, Desando S, et al. Interleukin-4 and interleukin-13 cause barrier dysfunction in human airway epithelial cells. Tissue Barriers. 2013;1:e24333.
- Choudhury P, Biswas S, Singh G, et al. Immunological profiling and development of a sensing device for detection of IL-13 in COPD and asthma. Bioelectrochemistry (Amsterdam, Netherlands). 2022:143, 107971–107971.
- Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007;119:1303-10.
- Doyle AD, Mukherjee M, LeSuer WE, et al. Eosinophil-derived IL-13 promotes emphysema. Eur Respir J. 2019;53:1801291.
- Rabe KF, Rennard S, Martinez FJ et al. Targeting Type 2 Inflammation and Epithelial Alarmins in Chronic Obstructive Pulmonary Disease: A Biologics Outlook. Am J Respir Crit Care Med. 2023;208:395-405.
- St Georges' University London. St. George's Respiratory Questionnaire for COPD patients (SGRQ-C). 2003. Available at https://www.sgul.ac.uk/research/research-operations/research-administration/st-georges-respiratory-questionnaire/docs/SGRQ-C-English-version.pdf. Accessed 23/07/2024.
MAT-XU-2402876 (v3.0) Date of Preparation: March 2025