- Article
- Source: Campus Sanofi
- 23 Oct 2023
ESC/EAS DYSLIPIDAEMIA GUIDELINE INTERACTIVE (20 MINUTES)
Lipoproteins, dyslipidaemia and ASCVD
Lipoproteins
Most cholesterol is synthesised in the liver, where it is packaged with triglycerides to form lipoproteins for transport around the bloodstream. Lipoproteins are used for energy utilisation, lipid deposition, steroid hormone production and bile acid formation.
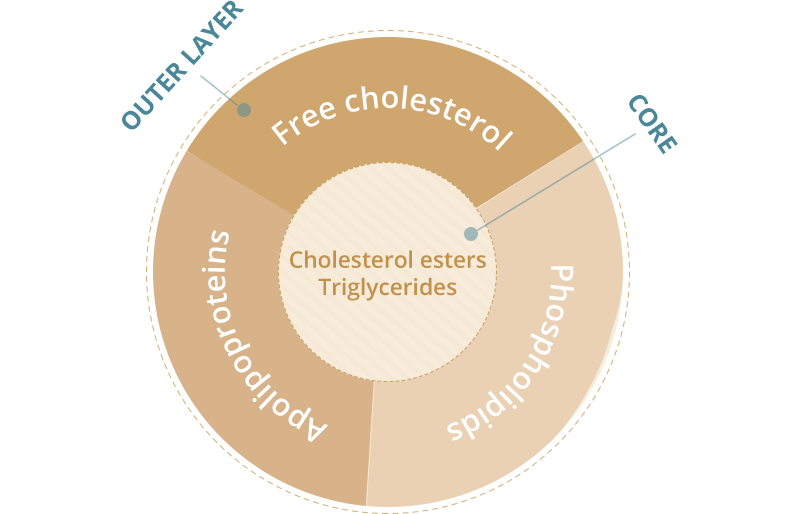
Composition of lipoproteins
Apolipoproteins (ApoB, ApoA1, etc.) are protein components that act as:
- structural components
- ligands for cellular receptor binding
- enzyme activators or inhibitors
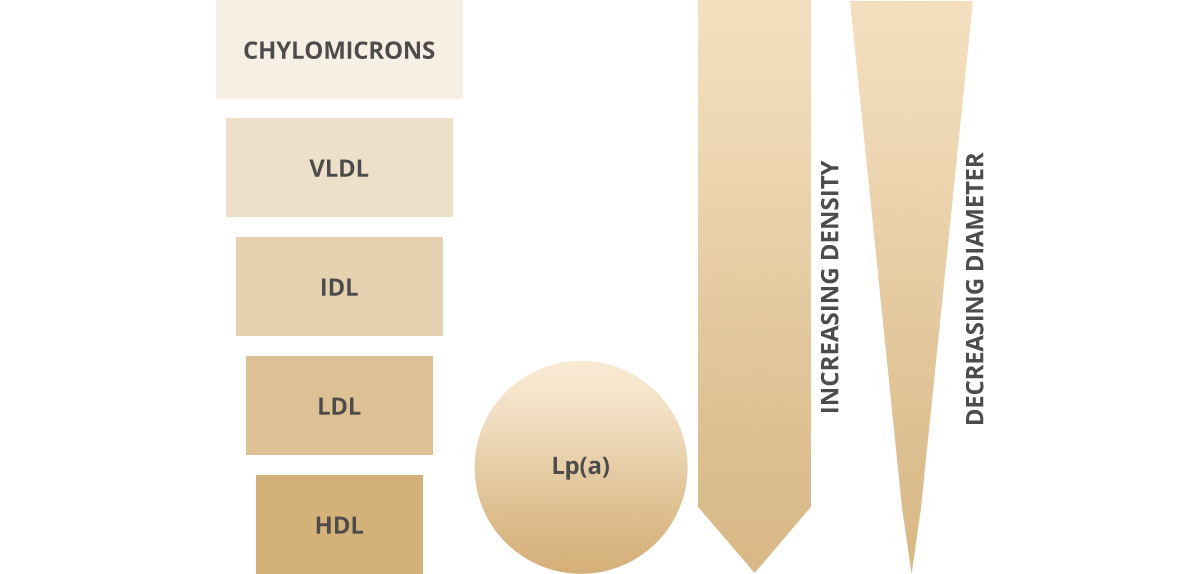
6 major lipoproteins in blood
Lipoproteins are gradually hydrolysed to release TG for energy storage and consumption, becoming less dense as a result.
Graphic adapted from 2019 ESC Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. European Heart Journal 2019; 41(1): 111-188
Dyslipidaemia and risk of ASCVD
Dyslipidaemia is a group of lipid and lipoprotein abnormalities, including high fasting and post-prandial TG, ApoB, and small dense LDL, and low HDL-C and ApoA1 levels. Increased LDL-C raises the risk of ASCVD.
ASCVD
CVD, of which ASCVD is the major component, is responsible for >4 million deaths in Europe each year (2.2 million women and 1.8 million men); CV deaths before the age of 65 years are more common in men (490,000 vs 193,000).
Prevention of ASCVD
There is no longer an ‘LDL-C’ hypothesis, but established facts that increased LDL-C values are causally related to ASCVD. Lowering LDL-C as much as possible reduces the risk of future CV events.
ASCVD prevention in the general population starts with promoting healthy lifestyle behaviour; individuals must tackle unhealthy lifestyles and reduce causal CV risk factors, such as LDL-C or blood pressure.
Prevention of ASCVD in a given person should relate to his or her total CV risk: the higher the risk, the more intense the action should be.
Managing dyslipidaemia
There are three steps in the management and treatment of dyslipidaemia:

Section abbreviations
ApoA1, apolipoprotein-A1; ApoB, apolipoprotein-B; ASCVD, atherosclerotic CVD; CV, cardiovascular; CVD, cardiovascular disease; HDL, high density lipoprotein; HDL-C, HDL cholesterol; IDL, intermediate density lipoprotein; LDL, low density lipoprotein; LDL-C, LDL cholesterol; Lp(a), lipoprotein(a); TG, triglycerides; VLDL, very low density lipoprotein.
Risk assessment
The strongest driver of total CV risk is age, which can be considered as ‘exposure time’ to risk factors.
- Evaluate clinical risk factors
- Total CV risk is very high or high if the patient has any of:
- Documented ASCVD
- T1DM or T2DM
- CKD
- Carotid or femoral plaques (detected by cardiovascular imaging)
- Very high levels of individual risk factors e.g. high TC, high systolic BP
These patients do not need a risk estimation model: they all need immediate, active management of all risk factors
- Total CV risk is very high or high if the patient has any of:
- Formal risk estimate
- In apparently healthy persons, CVD risk is usually the result of multiple, interacting risk factors which combine to give total CV risk estimation.
- Recommended: risk factor screening, including establishing the lipid profile, should be considered in men >40 years old, and in women >50 years of age or post-menopausal.
- A formal system such as SCORE estimates the 10-year cumulative risk of first fatal atherosclerotic event, and may help avoid under- and overtreatment.
- Young people (<40 years old) may have low absolute risk but high relative risk requiring at least intensive lifestyle advice. Older people, particularly male, may have estimated cumulative CV death risks exceeding 5-10% due to age alone, even when other CV risk factors are relatively low: therefore clinicians should carefully evaluate patients before initiating treatment in the elderly.
- Consider additional modifiers
- Risk is increased with every additional modifying factor:
- Social deprivation
- Obesity/central obesity
- Major psychiatric disorders
- Psychosocial stress including vital exhaustion
- Non-alcoholic fatty liver disease
- HIV treatment
- Chronic IMID
- Atrial fibrillation
- Obstructive sleep apnoea
- LVH
- CKD
- Physical inactivity
Non-invasive imaging can detect atherosclerotic vascular damage
- Risk is increased with every additional modifying factor:
SCORE
- The SCORE charts provide an estimate of a patient’s 10-year risk of death based on age, gender, smoking status, BP and TC
- HeartScore is an electronic version of SCORE that also takes HDL-C into account
- The charts are for people without overt CVD, T1DM, T2DM, CKD, FH, or very high levels of individual risk factors
- There are two charts, high risk and low risk: the chart selected depends on the CVD mortality experience in the particular country (according to WHO data from the Global Burden of Disease study)
- High-risk countries have an age-adjusted CVD mortality rate of >150/100,000 (note: for countries with CV mortality rate of >350/100,000, the high-risk chart may underestimate risk)
- Low-risk countries have an age-adjusted adjusted CVD mortality rate of <150/100,000
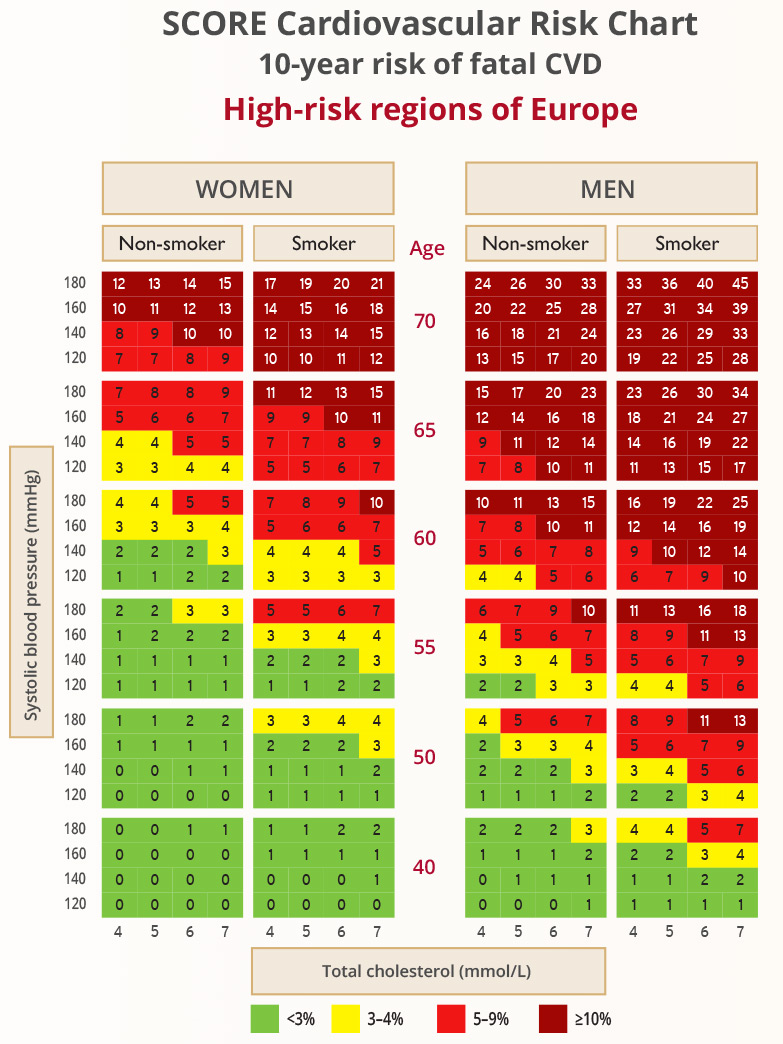
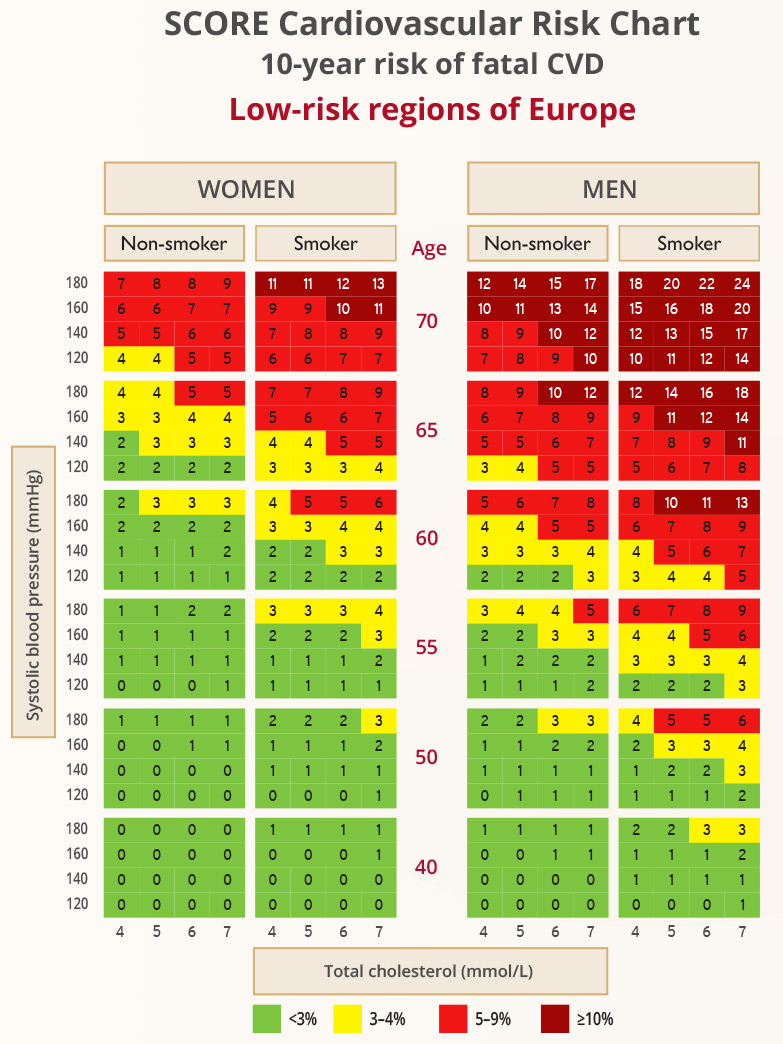
Graphic adapted from 2019 ESC Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. European Heart Journal 2019; 41(1): 111-188
Imaging
- Non-invasive CV imaging techniques can detect the presence, estimate the extent, and evaluate the clinical consequences of atherosclerotic vascular damage
- Imaging should be considered for complete classification of total CVD risk
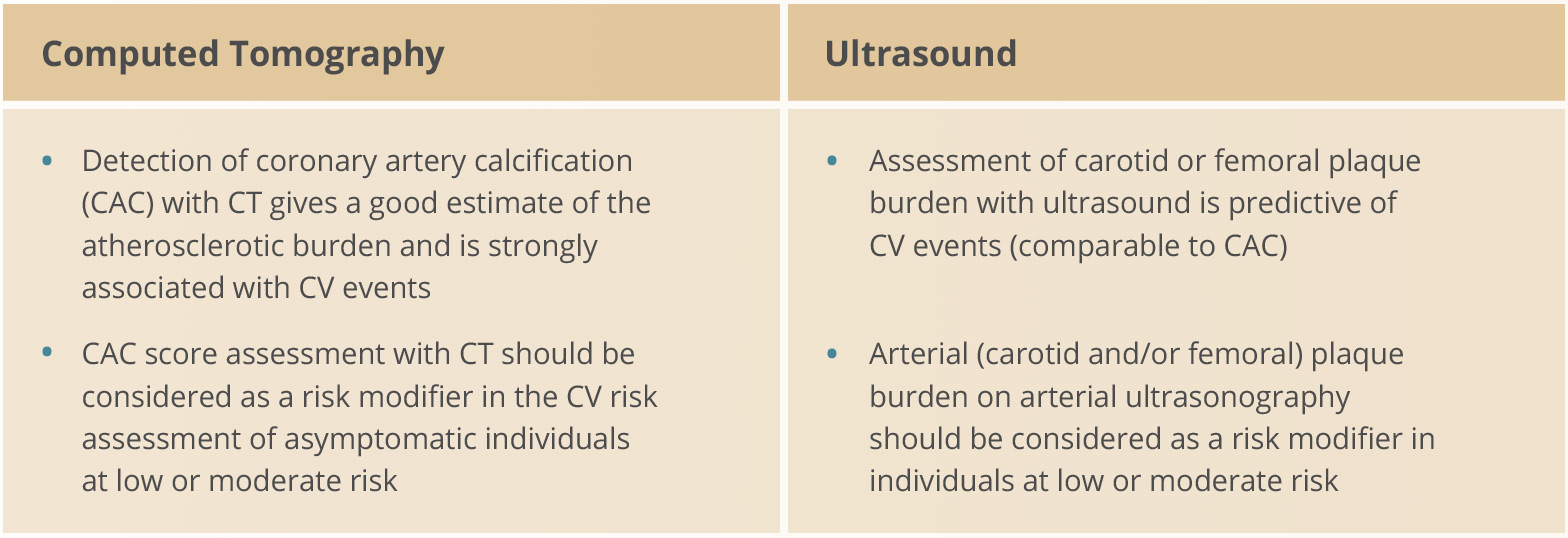
Graphic adapted from 2019 ESC Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. European Heart Journal 2019; 41(1): 111-188
Measuring plasma lipids and lipoproteins
- Measurements of lipids and lipoproteins are used to estimate the risk of ASCVD and guide therapeutic decision-making
- Recent studies suggest that fasting is not necessary for lipid measurement

Graphic adapted from 2019 ESC Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. European Heart Journal 2019; 41(1): 111-188
Section abbreviations
ApoA, apolipoprotein-A; ApoB, apolipoprotein-B; ASCVD, atherosclerotic CVD; BP, blood pressure; CAC, coronary artery calcification; CKD, chronic kidney disease; CT, computed tomography; CV, cardiovascular; CVD, cardiovascular disease; DM, diabetes mellitus; FH, familial hypercholesterolaemia; HDL, high density lipoprotein; HDL-C, HDL cholesterol; HIV, human immunodeficiency virus; IMID, immune-mediated inflammatory disorder; LDL, low density lipoprotein; LDL-C, LDL cholesterol; Lp(a), lipoprotein(a); LVH, left ventricular hypertrophy; SCORE, Systematic Coronary Risk Estimation; T1DM, type 1 DM; T2DM, type 2 DM; TC, total cholesterol; TG, triglycerides; WHO, World Health Organization.
Cardiovascular risk categories
A total CV risk estimate is part of a continuum: the cut-off points used to define high risk are part arbitrary and part based on risk levels at which benefit is evident in clinical trials. High risk patients should be identified and managed; patients at moderate risk should also receive lifestyle advice and may need drug therapy.
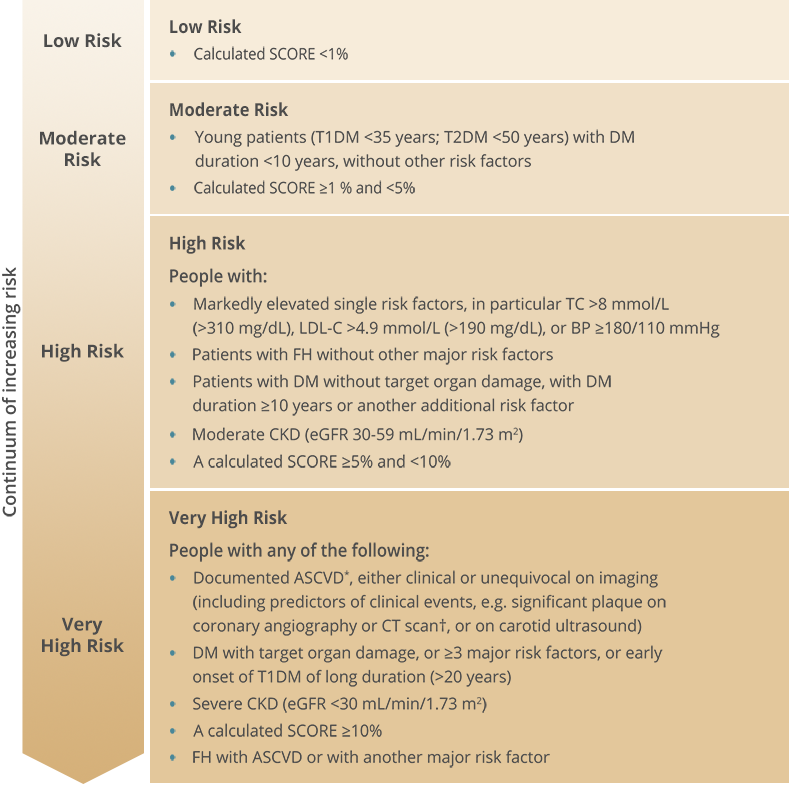.png)
* Includes previous acute coronary syndrome (myocardial infarction or unstable angina), stable angina, coronary revascularization (PCI, CABG, and other arterial revascularization procedures), stroke and TIA, and peripheral arterial disease
† Multivessel coronary disease with two major epicardial arteries having >50% stenosis
Graphic adapted from 2019 ESC Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. European Heart Journal 2019; 41(1): 111-188
Intervention strategies as a function of total
CV risk and untreated LDL-C levels
Prevention of ASCVD should relate to the person's total CV risk: the higher the risk, the more intense the action should be. The total risk approach allows flexibility; if optimal control cannot be achieved with one risk factor, trying harder with the other factors can still reduce risk.
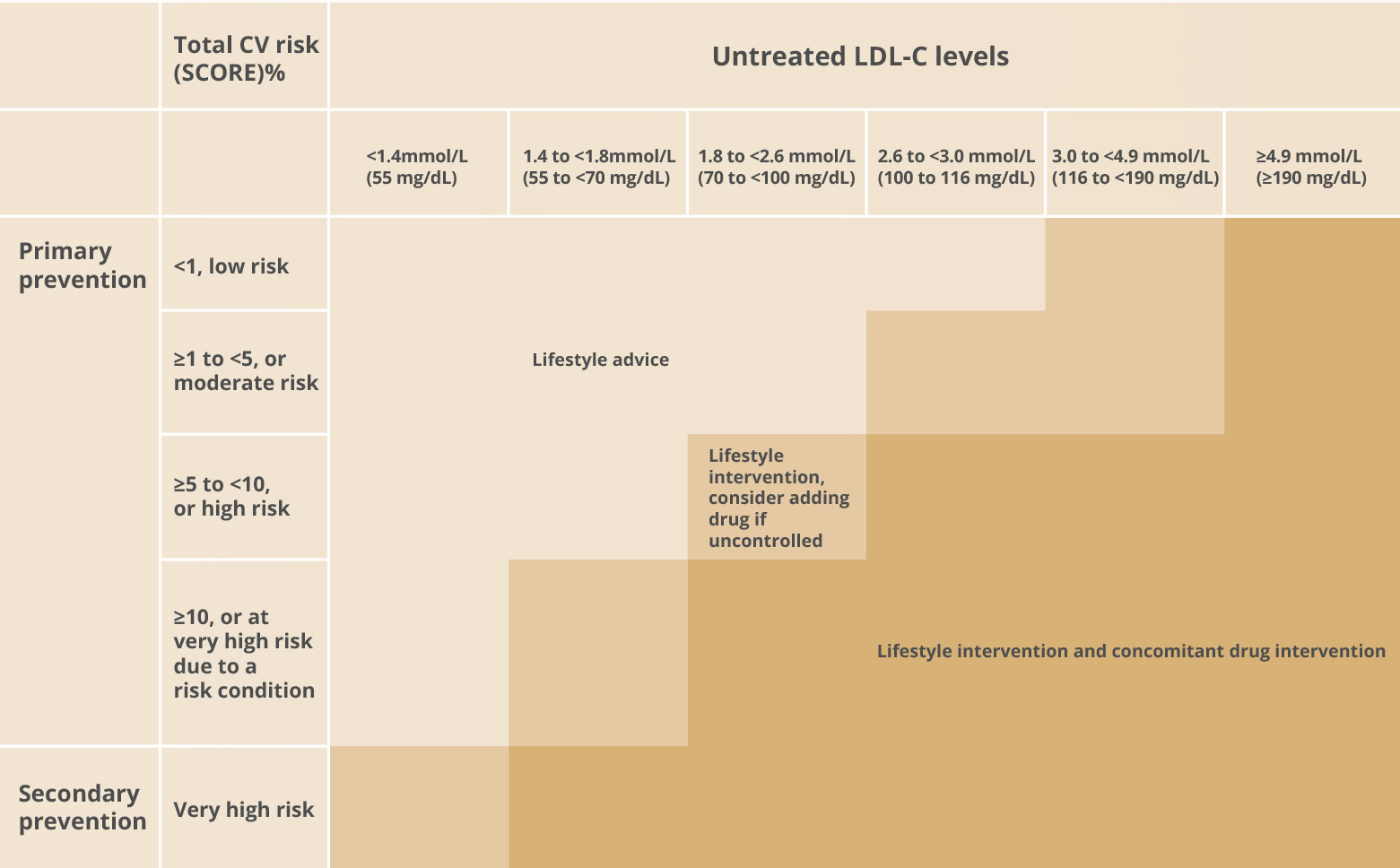
Graphic adapted from 2019 ESC Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. European Heart Journal 2019; 41(1): 111-188
Section abbreviations
ASCVD, atherosclerotic cardiovascular disease; BP, blood pressure; CABG, coronary artery bypass graft surgery; CKD, chronic kidney disease; CT, computed tomography; CV, cardiovascular; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FH, familial hypercholesterolaemia; LDL-C, low density lipoprotein cholesterol; PCI, percutaneous coronary intervention; SCORE, Systematic Coronary Risk Estimation; T1DM, type 1 DM; T2DM, type 2 DM; TC, total cholesterol; TIA, transient ischaemic attack.
Treating dyslipidaemia reduces CV risk
Increased LDL-C raises the risk of ASCVD. Lowering LDL-C reduces the risk of future CV events.
.png)
- The greater the absolute LDL-C reduction, the greater the CV risk reduction
- The benefits related to LDL-C reduction are not specific to statin therapy but can be gained by any successful method or treatment
- There is no level of LDL-C below which benefit ceases or harm occurs has been defined
.png)
- Variability: as individuals respond differently to drug treatments, a tailored approach to management is needed
- CV risk reduction should be individualized
- Setting a treatment goal may facilitate adherence
Treatment goals
Treatment goals are tailored to the total CV risk level: the higher the risk, the lower the target LDL-C.
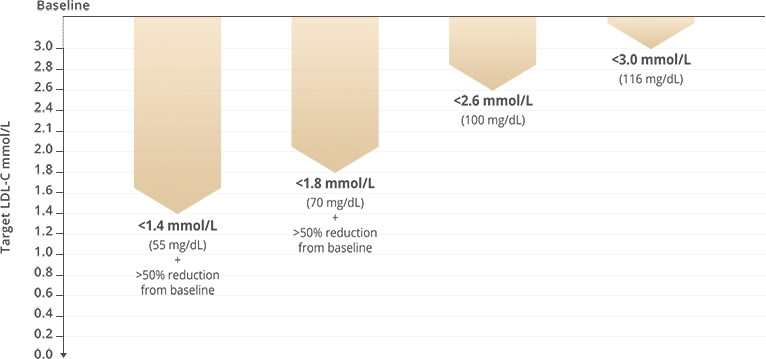
- ASCVD (clinical or imaging)
- SCORE ≥10%
- FH with ASCVD or with another major risk factor
- Severe CKD (eGFR <30 mL/min)
- DM with target organ damage; ≥3 major risk factors; or early onset of T1DM of long duration (>20 years)
- SCORE ≥5% and <10%
- Markedly elevated single risk factors, in particular TC >8 mmol (310 mg/dL) or LDL-C >4.9 mmol/L (190 mg/dL) or BP ≥180/110 mmHg
- FM without other major risk factors
- Moderate CKD (eGFR 30–59 mL/min)
- DM without target organ damage, with DM duration ≥10 years or other additional risk factor
- SCORE ≥1% and <5%
- Young patients (T1DM <35 years; T2DM <50 years) with DM duration <10 years without other risk factors
- SCORE <1%
- ASCVD (clinical or imaging)
- SCORE ≥10%
- FH with ASCVD or with another major risk factor
- Severe CKD (eGFR <30 mL/min)
- DM with target organ damage; ≥3 major risk factors; or early onset of T1DM of long duration (>20 years)
- SCORE ≥5% and <10%
- Markedly elevated single risk factors, in particular TC >8 mmol (310 mg/dL) or LDL-C >4.9 mmol/L (190 mg/dL) or BP ≥180/110 mmHg
- FM without other major risk factors
- Moderate CKD (eGFR 30–59 mL/min)
- DM without target organ damage, with DM duration ≥10 years or other additional risk factor
- SCORE ≥1% and <5%
- Young patients (T1DM <35 years; T2DM <50 years) with DM duration <10 years without other risk factors
- SCORE <1%

Graphic adapted from 2019 ESC Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. European Heart Journal 2019; 41(1): 111-188
Section abbreviations
ASCVD, atherosclerotic cardiovascular disease; CV, cardiovascular; LDL-C, low density lipoprotein cholesterol.
Lifestyle modifications
Dietary factors influence the development of CVD
- Dietary factors are key for both development and prevention of ASCVD, either directly or via their action on traditional risk factors such as plasma lipids, blood pressure or glucose levels
- High saturated fat intake increases LDL-C
- Higher consumption of fruit, non-starchy vegetables, nuts, legumes, fish, vegetable/olive oils, yoghurt, wholegrains (such as the Mediterranean and DASH diets) with lower consumption of red and processed meats, refined carbohydrates and salt, is associated with a lower incidence of CV events
Lifestyle modifications that lower TC, LDL-C, and TG-rich lipoproteins, and increase HDL-C

Dietary: avoid or reduce
-
Dietary trans fats, saturated fats,
and cholesterol - Dietary carbohydrates and
mono- and disaccharides - Alcohol intake

Dietary: increase
- Dietary fibre

Dietary: use food supplements
- Functional foods enriched with phytosterols
- Red yeast rice neutraceuticals
- N-3 polyunsaturated fats

Lose excess weight

Increase physical activity

Stop smoking
Section abbreviations
ASCVD, atherosclerotic CVD; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides.
Pharmacological treatments
Statins
Recommendation: use a high-intensity statin at the highest tolerable dose to reach the treatment goal
- Statins reduce cholesterol synthesis in the liver by competitively inhibiting the enzyme HMG-CoA reductase, the rate-limiting step in cholesterol biosynthesis
- The degree of LDL-C reduction is dose-dependent and varies between the different statins
- A high-intensity statin regimen: the dose that, on average, reduces LDL-C by ≥50%; a moderate-intensity statin regimen: the dose expected to reduce LDL-C by 30-50%
- There is considerable interindividual variation in LDL-C reduction with the same dose of drug: poor response to statin treatment may be due to poor compliance, but also may be due to genetic background. Up-titration may be required
- Effect on CV mortality and morbidity: statin/more statin reduces major vascular events
(MI, CAD death, or any stoke or coronary revascularisation) by ~22%, major corony events by 23%, CAD death by 20%, total stroke by 17%, and total mortality by 10% over 5 years for each 1mmol/L reduction in LDL-C - Safety: interactions with concomitant drug therapies may increase the risk of AEs. The most clinically relevant AE is myopathy. Others include mild elevation of ALT in the liver or increased risk of new-onset DM.
Cholesterol absorption inhibitors
Recommendation: if the treatment goal is not reached with high-intensity statin, add ezetimibe
- Ezetimibe inhibits intestinal uptake of dietary and biliary cholesterol at the level of the brush border of the intestine without affecting the absorption of fat-soluble nutrients. The amount of cholesterol delivered to the liver is reduced; the liver then upregulates LDLR expression, increasing clearance of LDL from the blood
- When added to ongoing statin therapy in clinical trials, ezetimibe reduced LDL-C by an additional 21–27%*
- Relatively high interindividual variation in LDL-C reduction
- Effect on CV mortality and morbidity: adding ezetimibe to simvastatin in patients after ACS reduced CV events compared with simvastatin alone (32.7% vs 34.7%, p=0.016). Ischaemic stroke was reduced by 21% (p=0.008)
- Safety: no clinically significant effects of age, sex, or race on ezetimibe pharmacokinetics. No dose adjustment needed in patients with mild hepatic impairment or mild-to-severe renal insufficiency.
* Compared with placebo, in patients with hypercholesterolaemia, with or without established coronary heart disease
PCSK9 inhibitors
Recommendation: if the treatment goal is not reached with high-intensity statin + ezetimibe, add PCSK9 inhibitor
- PCSK9 is involved in control of the LDLR: elevated PCSK9 reduces LDLR expression and leads to increased plasma LDL. Reducing PCSK9 levels or function using mAbs leads to lower plasma LDL-C
- Data suggests there are greater reductions in LDL-C when these mABs (PCSK9 inhibitors) are combined with statins, which appear to increase circulating serum PCSK9.
- Average reduction in LDL-C is 60% (depending on dose) either alone or in combination with statins and/or other lipid-lowering therapies
- Efficacy appears to be independent of any background therapy
- PCSK9 inhibition + ezetimibe reduced LDL-C in patients in whom statins could not be prescribed
- PCSK inhibitors reduced LDL-C in patients at high CV risk, including those with DM
- In clinical trials, PCSK9 inhibitors also reduced TG and Lp(a), and increased HDL-C and ApoA1
- Effect on CV mortality and morbidity: early preliminary data from phase III trials suggest reduced CV events in line with LDL-C reduction
- Safety profile: Among the most frequently reported AEs are local injection site reactions, upper respiratory tract signs and symptoms and pruritus.
Treatment pathway for pharmacological
lowering of LDL-C
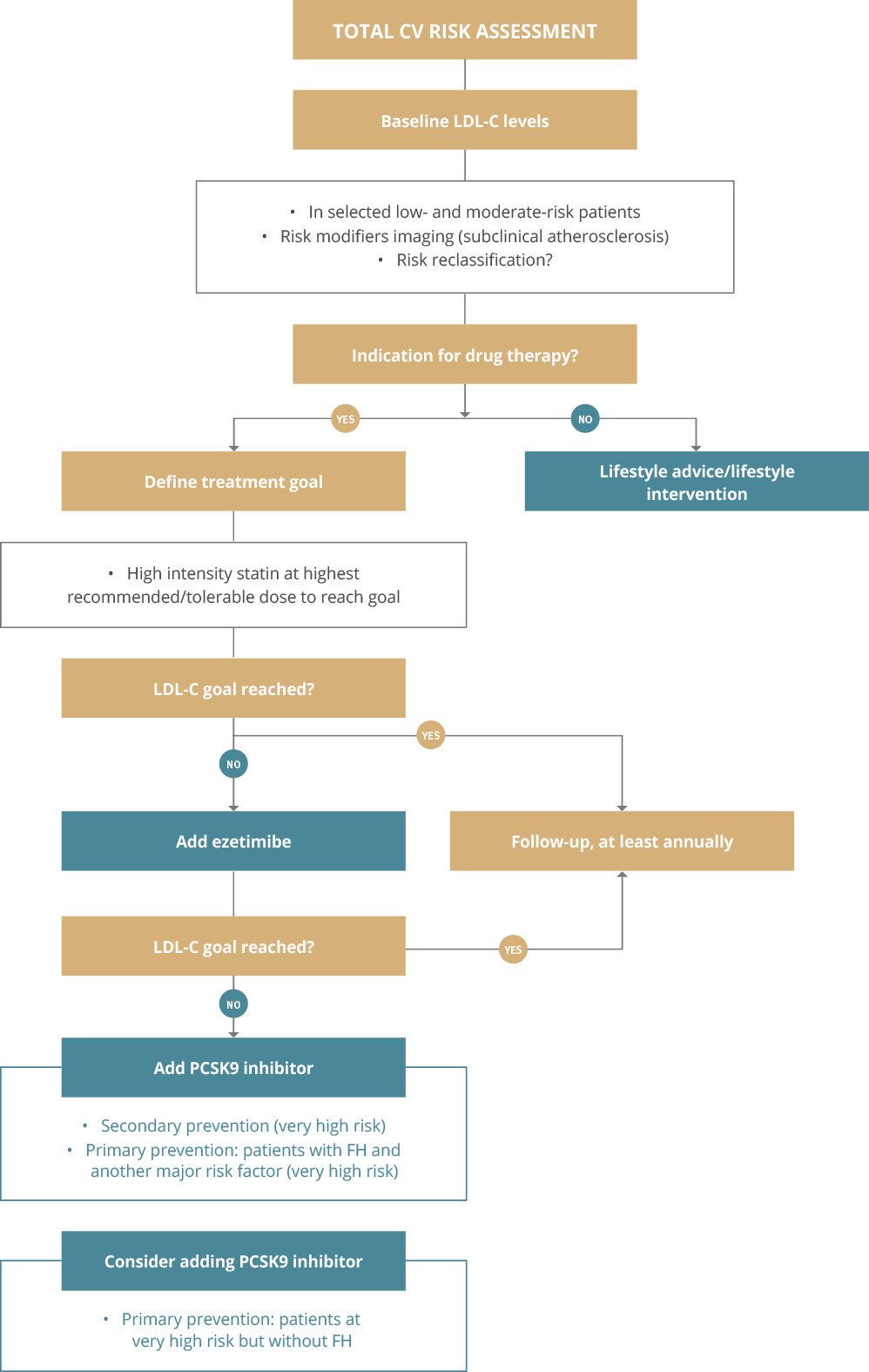
* Please refer to the "risk table" for further information. PSCK9i are licensed for patients with established CVD.
Please refer to individual SmPCs for further information.
Graphic adapted from 2019 ESC Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. European Heart Journal 2019; 41(1): 111-188
Section abbreviations
ACS, acute coronary syndrome; AE, adverse event; ALT, alanine aminotransferase; ApoA1, apolipoprotein-A1; CAD, coronary artery disease; CV, cardiovascular; DM, diabetes mellitus; HMG-CoA, hydroxymethylglutaryl-coenzyme A; LDL, low density lipoprotein; LDL-C, LDL cholesterol; LDLR, LDL receptor; Lp(a), lipoprotein(a); mABs, monoclonal antibodies; MI, myocardial infarction; PCSK9, proprotein convertase subtilisin/kexin type 9; TG, triglycerides.
Considerations for managing dyslipidaemia in patients with ACS
Patients with ACS
- Patients with ACS are at increased risk of experiencing recurrent CV events
- As well as lipid management, patients should engage in comprehensive risk reduction including lifestyle changes, risk factor management and implementation of cardioprotective drug strategies
- Where possible, patients should be on a cardiac rehabilitation programme to enhance lipid control
- Attainment of LDL-C target values remains suboptimal in this very high-risk setting
Treatment
- High dose statin therapy should be started/continued as early as possible, regardless of initial LDL-C levels, in all ACS patients without any contraindications or definite history of intolerance
- 4-6 weeks after ACS: check lipid levels to see if treatment goal achieved and address safety issues - adapt statin treatment accordingly
- If LDL-C goal is not reached after 4-6 weeks of maximum tolerated dose statin, add ezetimibe
- If LDL-C goal is not reached after 4-6 weeks of statin + ezetimibe, add PCSK9 inhibitor
- Consider ezetimibe in patients intolerant of or with contraindications to statins
- For patients presenting with an ACS and with LDL-C levels not at goal despite already taking maximum tolerated dose statin + ezetimibe, consider adding a PCSK9 inhibitor early after the event (during hospitalisation if possible)
Section abbreviations
ACS, acute coronary syndrome; CV, cardiovascular; LDL-C, low density lipoprotein cholesterol; PCSK9, proprotein convertase subtilisin/kexin type 9.
Considerations for managing dyslipidaemia in patients with familial dyslipidaemia
Familial dyslipidaemia
- Plasma lipid levels are largely determined by genetic factors
- This can manifest as familial dyslipidaemia
- FH is the most common monogenic lipid disorder and is strongly related to CVD
Treatment
- Cholesterol-lowering treatment should be initiated as soon as possible after a diagnosis has been made
- Initiate high-intensity statin, in most cases in combination with ezetimibe
- LDL-C goals are usually ≥50% reduction from baseline, with LDL-C <1.4mmol/L (<55mg/dL) if at very high risk of ASCVD, or <1.8mmol/L (<70mg/dL) if no ASCVD or other major risk factor
- PCSK9 inhibitors are recommended in very-high-risk patients if the treatment goal is not achieved on maximum tolerated statin + ezetimibe
- PCSK9 inhibitors are recommended in patients who cannot tolerate statins
- Treat children with a statin from age 8‐10 years and educate on proper diet
- Imaging to detect asymptomatic atherosclerosis is recommended
Please refer to individual SmPCs for further information
Section abbreviations
ASCVD, atherosclerotic CVD; CVD, cardiovascular disease; FH, familial hypercholesterolaemia; LDL-C, low density lipoprotein cholesterol; PCSK9, proprotein convertase subtilisin/kexin type 9, SmPC, summary of product characteristics.
Considerations for managing dyslipidaemia in patients with diabetes
Patients with diabetes
- CVD is the leading cause of morbidity and mortality in patients with T2DM
- DM is an independent risk factor for CVD
- T1DM is associated with high CVD risk
- LDL-C is the primary target of lipid-lowering therapy
Treatment
- LDL-C treatment goals for T2DM patients are the same as for patients without T2DM
- First line therapy is statins
- If treatment goal not reached, intensify statin therapy before introducing combination therapy
- If treatment goal not reached, consider statin-ezetimibe combination therapy
- Statin therapy is not recommended in patients with DM who are considering pregnancy or not using adequate contraception
- Statin therapy may be considered in T1DM and T2DM patients aged ≤30 years with evidence of end organ damage and/or an LDL-C level >2.5mmol/L, as long as pregnancy is not being planned
Section abbreviations
CVD, cardiovascular disease; DM, diabetes mellitus; LDL-C, low density lipoprotein cholesterol; T1DM, type 1 DM; T2DM, type 2 DM.
Considerations for managing dyslipidaemia in elderly patients
Elderly people
- Use of statin therapy declines with age, reflecting differences in prescription and compliance
- Safety and adverse effects of statins, particularly statin-drug interactions, are of concern because of increased comorbidities, multiple medications and altered pharmacokinetics/pharmacodynamics
Treatment
- Treatment with statin is recommended for elderly people with ASCVD in the same way as for younger patients
- Treatment with statins is recommended for primary prevention, according to the level of risk, in elderly people aged ≤75 years
- Initiation of statins for primary prevention in elderly people aged >75 years may be considered, if at high or very high risk
- If there is significant renal impairment and/or potential for drug interactions, start statin at low dose and titrate upwards to achieve LDL-C treatment goals
Section abbreviations
ASCVD, atherosclerotic CVD; CVD, cardiovascular disease; LDL-C, low density lipoprotein cholesterol.
© The European Society of Cardiology and the European Atherosclerosis Association 2019. All rights reserved. For permissions please email: journals.permissions@oup.com.
This publication is for personal and educational use only. No commercial use is authorized. No part of this publication or the original ESC/EAS Guidelines from which it is derived may be translated or reproduced in any form without written permission from the ESC. Permission may be obtained upon submission of a written request to Oxford University Press, the publisher of the European Heart Journal and the party authorized to handle such permissions by the ESC. Sanofi has obtained permission to publish this material and to distribute it to health professionals within France, Spain, IE, Norway, Greece, Croatia, Slovenia, United Kingdom.
Disclaimer:
The ESC/EAS Guidelines represent the views of the ESC/EAS and were produced after careful consideration of the scientific and medical knowledge and the evidence available at the time of their dating. The ESC/EAS is not responsible in the event of any contradiction, discrepancy and/or ambiguity between the ESC/EAS Guidelines and any other official recommendations or guidelines issued by the relevant public health authorities, in particular in relation to good use of health care or therapeutic strategies. Health professionals are encouraged to take the ESC/EAS Guidelines fully into account when exercising their clinical judgment as well as in the determination and the implementation of preventive, diagnostic or therapeutic medical strategies. However, the ESC/EAS Guidelines do not override in any way whatsoever the individual responsibility of health professionals to make appropriate and accurate decisions in consideration of each patient’s health condition and in consultation with that patient and the patient's caregiver where appropriate and/or necessary. Nor do the ESC/EAS Guidelines exempt health professionals from taking careful and full consideration of the relevant official updated recommendations or guidelines issued by the competent public health authorities in order to manage each patient’s case in light of the scientifically accepted data pursuant to their respective ethical and professional obligations. It is also the health professional’s responsibility to verify the applicable rules and regulations relating to drugs and medical devices at the time of prescription.
Reproduced from: Mach F, Baigent C, Catapano AL, et al, 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS), European Heart Journal 2020; 41 (1): 111–188, doi:10.1093/eurheartj/ehz455. With permission of Oxford University Press on behalf the European Society of Cardiology.
Please visit: https://www.escardio.org/Guidelines/Clinical-Practice-Guidelines/Dyslipidaemias-Management-of
Sanofi was not involved in the development of this ESC Guideline and in no way influenced its content.
MAT-XU-2204595 (v3.0) Date of Preparation: October 2023
