- Article
- Source: Campus Sanofi
- 23 Jul 2025
ODYSSEY OUTCOMES Efficacy Praluent® (alirocumab)
.png)

In patients with a prior cv event, Praluent® (alirocumab) significantly reduced the risk of MACE (primary endpoint) in patients already on high intensity statins1,2
~90% of patients in ODYSSEY OUTCOMES were on high-intensity statins1
Study design: ODYSSEY OUTCOMES was a randomized, double-blind, placebo-controlled phase 3 study. Patients with a recent MI or unstable angina, and on high-intensity statin (40 or 80 mg atorvastatin or 20 or 40 mg rosuvastatin, or maximally tolerated dose of one of these agents) +/- other lipid-lowering therapy but not at predefined target LDL-C were enrolled.1
MACE
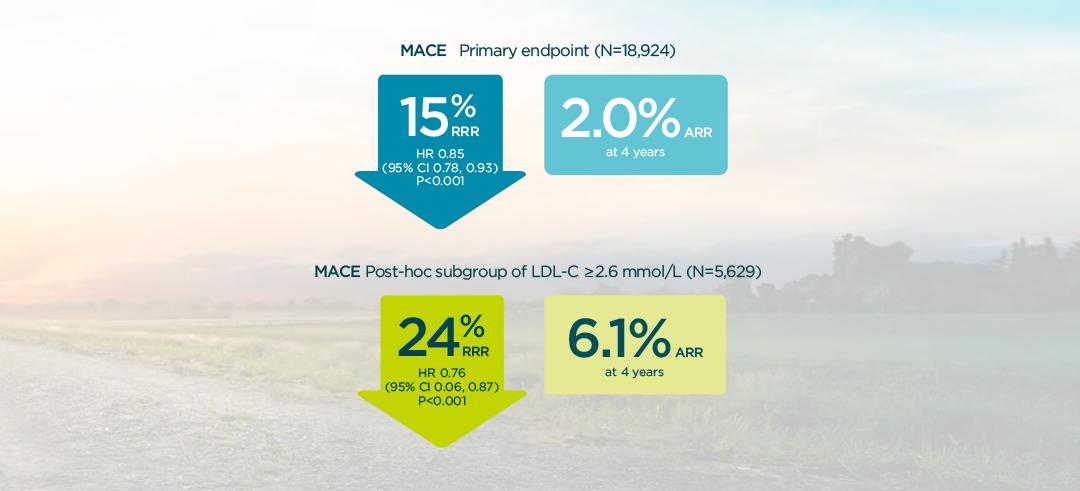
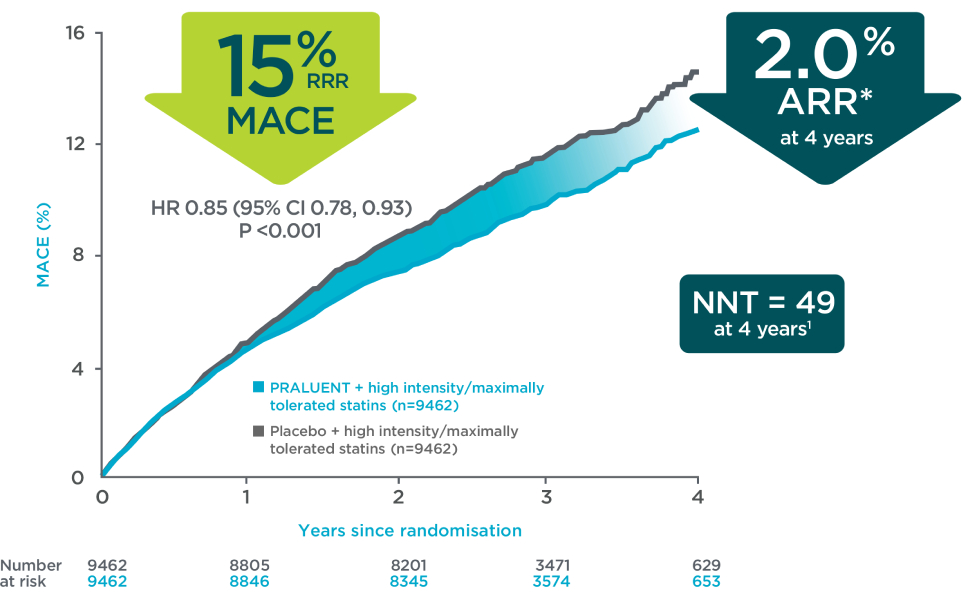
In patients with a prior CV event, Praluent® demonstrated a significant reduction in the risk of MACE for the overall trial population vs placebo, HR 0.85 [95% CI 0.78 - 0.93] p=0.0003).2
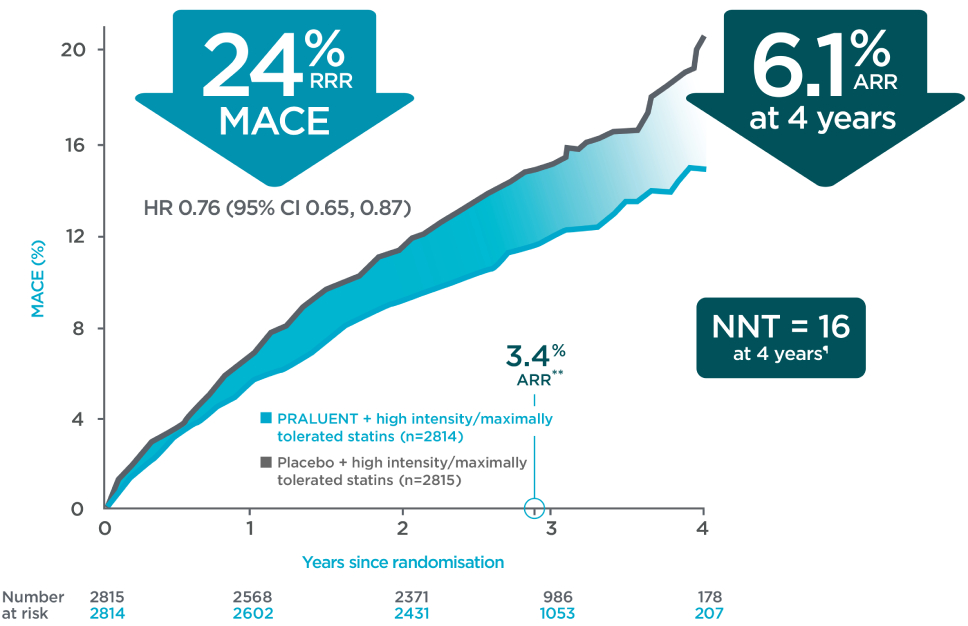
A greater absolute benefit was observed in patients with baseline ≥2.6 mmol/L LDL-C (non-prespecified analysis).2
See Kaplan-Meier curves for MACE
Praluent® demonstrated a significant reduction in the risk of MACE for the overall trial population.1 In post-ACS patients, Praluent® demonstrated a greater absolute benefit in MACE in patients with LDL-C ≥2.6 mmol/L, compared to the overall trial population1,2
Primary endpoint in overall population1,3
Primary endpoint in a post-hoc analysis of patients with ≥2.6 mmol/L2§
Adapted from Schwartz G, et al. 2018, and Schwartz G, et al. 2018 suppl appendix.
*Observed cumulative incidence of events over 4 years.
†Observed cumulative incidence of events over a median of 2.8 years.
§The effect of PRALUENT on the relative risk of the composite endpoint did not differ significantly between pre-specified LDL-C subgroups. Interaction P value, P=0.09.
¶The NNT of 16 is calculated in accordance with the formula NNT = 1/ARR, with a 4-year Kaplan-Meier estimate ARR of 6.1%.
**Observed cumulative incidence of events over a median of 2.8 years (post hoc analysis).
References:
1. Schwartz G, et al. N Engl J Med 2018;379:2097–2107.
2. Schwartz G, et al. N Engl J Med 2018;379:2097–2107. Supplementary Appendix.
3. Praluent Summary of Product Characteristics. Available at https://www.medicines.org.uk/emc/product/8093/smpc. Accessed July 2025.
4. Data on file. Clinical study report. Sanofi and Regeneron Pharmaceuticals. June 2018.
Overall efficacy in main CV outcomes
Praluent® provided consistent results across CV outcomes1
Among the main secondary endpoints, the risks of any CHD event (including CHD death, nonfatal MI, UA requiring hospitalisation, and an ischaemia-driven coronary revascularisation procedure), major CHD event (including death from CHD and nonfatal MI), CV event (including nonfatal MI and nonfatal ischaemic stroke), and a composite of death from any cause, nonfatal MI or nonfatal ischemic stroke were significantly lower among patients treated with Praluent® than those who received placebo.1
View table and forest plot of CV outcome results
CV outcomes with Praluent® in the overall population in ODYSSEY OUTCOMES1
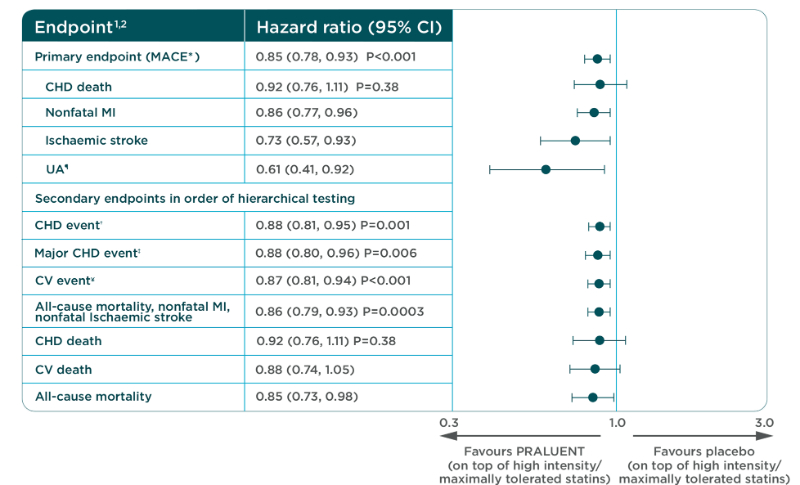
*MACE defined as a composite of: coronary heart disease (CHD) death, nonfatal myocardial infarction (MI), fatal and nonfatal ischaemic stroke, or unstable angina (UA) requiring hospitalisation.
†Unstable angina requiring hospitalisation.
‡CHD event defined as: major CHD event,§ UA requiring hospitalisation, ischaemia-driven coronary revascularisation procedure.
§Major CHD event defined as: CHD death, nonfatal MI.
||Cardiovascular event defined as follows: CV death, any nonfatal CHD event, and nonfatal ischaemic stroke.
References:
1. Praluent Summary of Product Characteristics. Available at https://www.medicines.org.uk/emc/product/8093/smpc. Accessed July 2025.
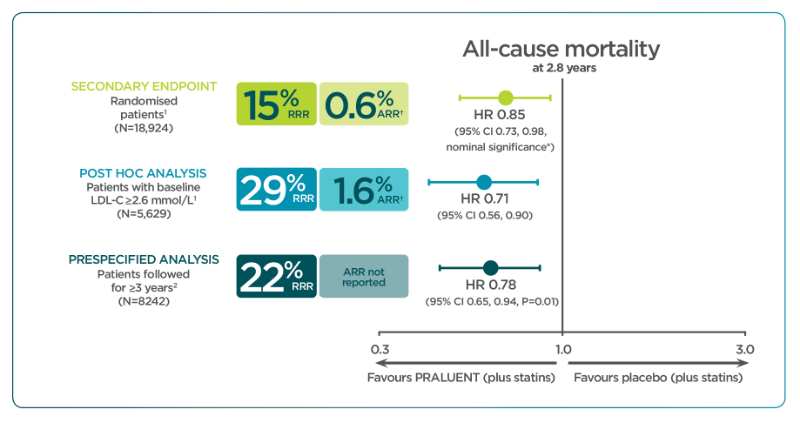
All-cause mortality
Praluent® was associated with a reduction of all-cause mortality (nominal significance by hierarchical testing) for the overall trial population vs placebo, HR 0.85 [95% CI 0.73–0.98]2,3.
In a post-hoc analysis, a greater benefit was observed in patients with baseline 100 mg/dL LDL-C for the overall trial population vs placebo, HR 0.71 [95% CI 0.56–0.90].2,3**
**Post-hoc analysis.
ACS=acute coronary syndrome; ARR=absolute risk reduction; CHD=coronary heart disease; CI=confidence interval; CV=cardiovascular; HCL-C=high-density lipoprotein cholesterol; HR=hazard ratio; LDL-C=low-density lipoprotein cholesterol; MACE=major adverse cardiac events (primary composite endpoint of CHD death, nonfatal myocardial infarction, fatal and nonfatal ischaemic stroke, or unstable angina requiring hospitalization); MI=myocardial infarction; NNT=number needed to treat; PCSK9i=proprotein convertase subtilisin/kexin type 9 inhibitor; RRR=relative risk reduction.
View forest plot for all-cause mortality
With PRALUENT, a reduction of all-cause mortality was observed with only nominal significance for the secondary endpoint
Adapted from Schwartz G, et al. 2018.
*This p value is considered nominally significant based on the pre-specified hierarchical analysis of secondary endpoints
†At end of trial after median follow-up of 2.8 years
References:
1. Schwartz G, et al. N Engl J Med 2018;379:2097–2107.
2. Steg G, et al. Circulation 2019;140(2):103-112.
3. Schwartz G, et al. N Engl J Med 2018;379:2097–2107. Supplementary Appendix.
Praluent®
Find more information on Indication, Administration and Mechanism of Action and watch videos about Praluent®.
.png)
References
- Schwartz G, et al. N Engl J Med 2018;379:2097–2107.
- Praluent Summary of Product Characteristics. Available at https://www.medicines.org.uk/emc/product/8093/smpc. Accessed July 2025.
- Schwartz G, et al. N Engl J Med 2018;379:2097–2107. Supplementary Appendix.
MAT-XU-2503024 (v1.0) Date of Preparation: July 2025