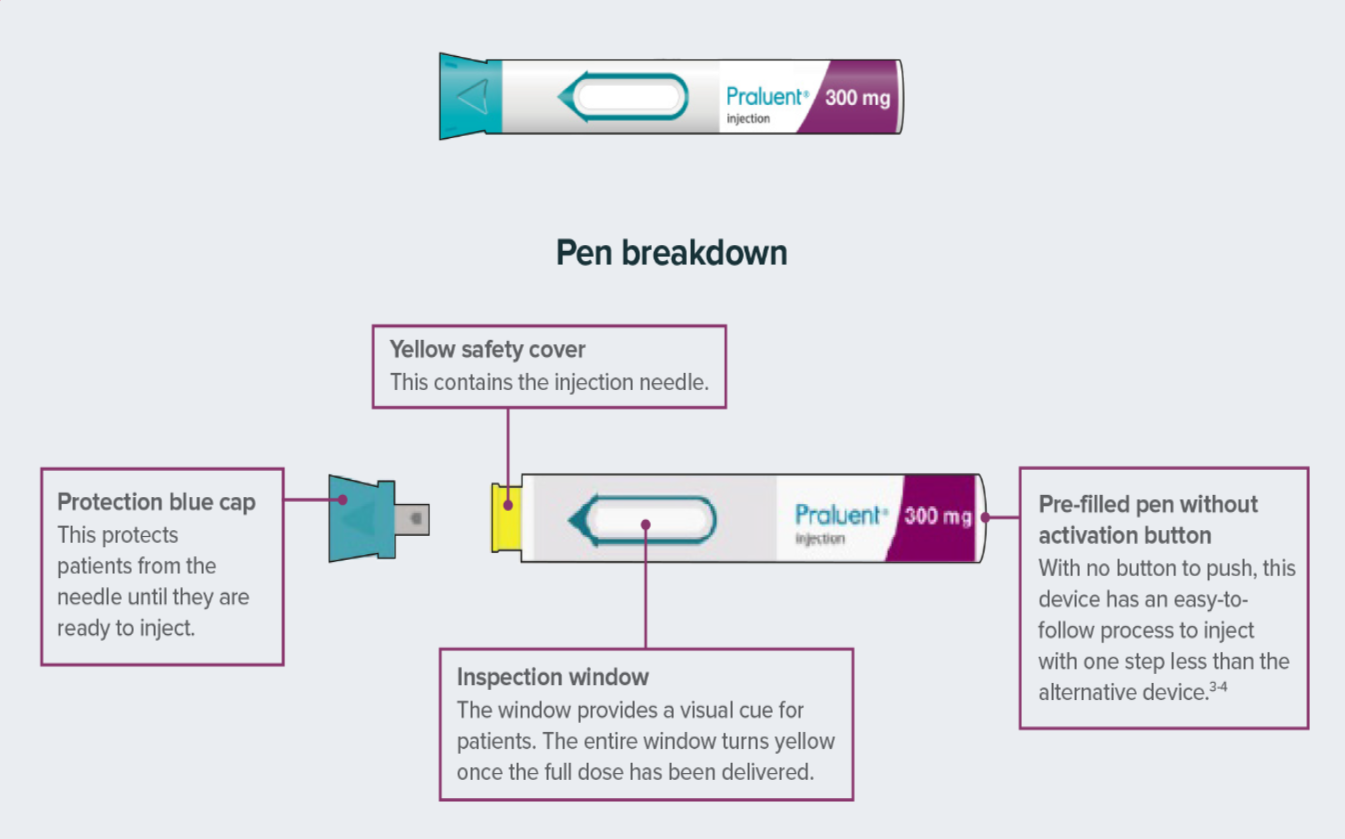
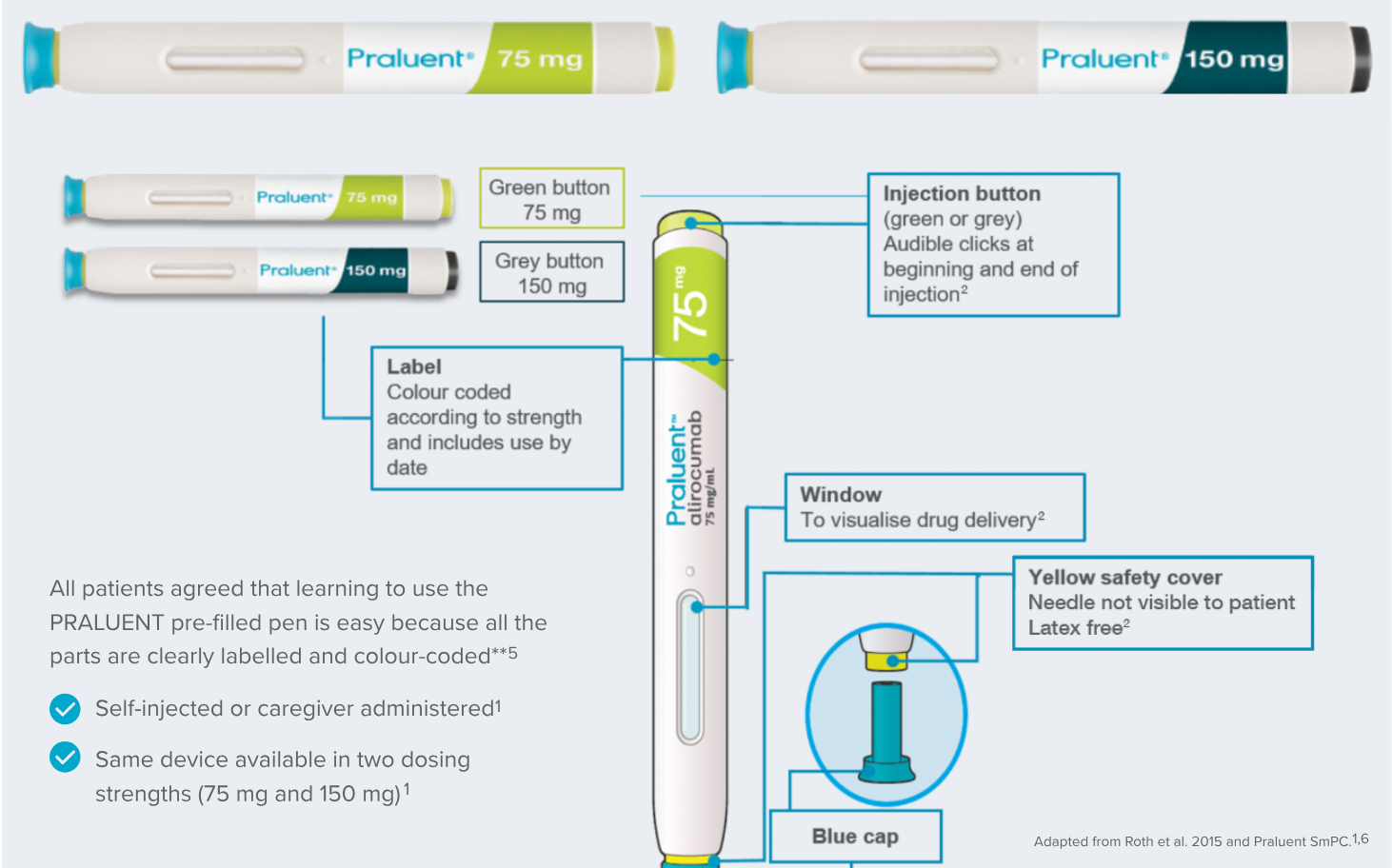
- Article
- Source: Campus Sanofi
- 23 Jul 2025
Praluent® (alirocumab) Administration


Praluent® can be injected subcutaneously into the thigh, abdomen or upper arm. Patients should rotate the injection site with each injection.1
Ease of use assessed as ≥9.8 out of 10 in all assessments for both devices*2
*Study of 69 patients who randomly received unsupervised, self-administered alirocumab 300 mg via 1 x 300 mg injection with the SYDNEY device (n=35) or 2 x 150 mg injections with the currently approved auto-injector (n=34). Possible answers ranged from 1 (very dissatisfied) to 10 (very satisfied).
**Evaluated in a cross-sectional, non-interventional study involving 151 patients enrolled in Praluent® phase 3 trials.
SmPC=summary of product characteristics.
Up to 30 days out of the fridge1
- Store in a refrigerator (2°C to 8°C). Do not freeze
- PRALUENT® can be kept outside a refrigerator below 25°C and protected from light for a single period not exceeding 30 days
- After removal from the refrigerator, the medicinal product must be used within 30 days or discarded.
- Keep the pen in the outer carton in order to protect from light.
Although many patients requiring lipid lowering therapies are not experienced with self-injected medication, in a real world study, results indicated that the pre-filled pen was well-accepted by patients.6
Dosage informationThe recommended alirocumab doses are 75 mg once every 2 weeks, 150 mg once every 2 weeks, 300 mg once every 4 weeks (monthly), administered subcutaneously. All doses may be used for initiation of treatment. The dose of alirocumab can be individualised based on patient characteristics such as baseline LDL-C level, goal of therapy, and response to treatment. Lipid levels can be assessed 4 to 8 weeks after treatment initiation or titration, and dose adjusted accordingly (up-titration or down-titration). Intense LDL-C reduction is expected with alirocumab 150 mg once every 2 weeks and 300 mg once every 4 weeks (monthly), where 150 mg once every 2 weeks is the maximum dose (see section 5.1 of the SmPC). |
Praluent®
Find more information on Indication, Administration and Mechanism of Action and watch videos about Praluent®.
.jpg)
References
- Praluent Summary of Product Characteristics. Available at https://www.medicines.org.uk/emc/product/8093/smpc. Accessed July 2025.
- Frias JP, Koren MJ, Loizeau V, et al. The SYDNEY Device Study: A Multicenter, Randomized, Open-label Usability Study of a 2-mL Alirocumab Autoinjector Device. Clinical Therapeutics. 2020; 42(1):94-107.
- Praluent Patient information lealfet 150mg. July 2025.
- Praluent Patient information leaflet 300mg. July 2025.
- Tatlock S, et al. Value Health 2017;20:430–440.
- Roth E, et al. Clin Ther 2015;37(9):1945–1954.
MAT-XU-2503036 (v1.0) Date of Preparation: July 2025