- Article
- Source: Campus Sanofi
- 23 Jul 2025
Dosing options – customise your patient’s treatment with Praluent® (alirocumab)
.

A 4-weekly (monthly) single injection
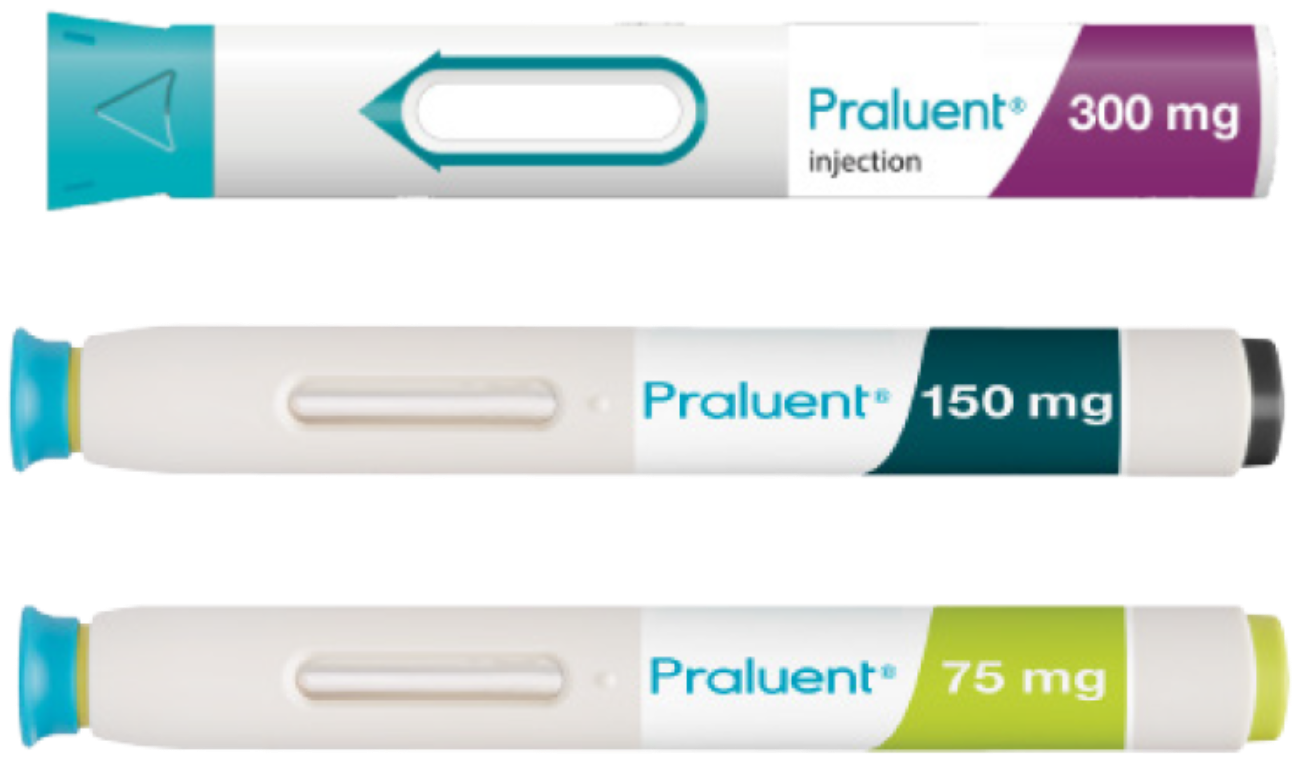
300 mg Q4W

A device you’ll recognise with one step less2,3
✅ No button to push
✅ Injection starts when device is pressed fully into the skin
✅ Designed to deliver once-monthly dose in ≤20 seconds4
Only PCSK9i with once-monthly single injection in a pre-filled pen1
✅ The potential to halve the administration days for your patients
Two twice-monthly options1
75 mg Q2W

150 mg Q2W

The recommended alirocumab doses are 75 mg once every 2 weeks, 150 mg once every 2 weeks, 300 mg once every 4 weeks (monthly), administered subcutaneously. All doses may be used for initiation of treatment.
Customised treatment with Praluent®
Dosage information
The dose of alirocumab can be individualised based on patient characteristics such as baseline LDL-C level, goal of therapy, and response to treatment. Lipid levels can be assessed 4 to 8 weeks after treatment initiation or titration, and dose adjusted accordingly (up-titration or down-titration). Intense LDL-C reduction is expected with alirocumab 150 mg once every 2 weeks, and 300 mg once every 4 weeks (monthly), where 150 mg once every 2 weeks is the maximum dose (see section 5.1 of the SmPC)
For full instructions on use refer to the Patient information Leaflet.
Praluent®
Find more information on Indication, Administration and Mechanism of Action and watch videos about Praluent®.
.jpg)
References
CV=cardiovascular; LDL-C=low-density lipoprotein cholesterol; Q2W=once every two weeks; Q4W=once every four weeks.
- Praluent Summary of Product Characteristics. Available at https://www.medicines.org.uk/emc/product/8093/smpc. Accessed July 2025.
- Praluent Patient information leaflet 150mg. July 2025.
- Praluent Patient information leaflet 300mg. July 2025.
- Frias JP, Koren MJ, Loizeau V, et al. The SYDNEY Device Study: A Multicenter, Randomized, Open-label Usability Study of a 2-mL Alirocumab Autoinjector Device. Clinical Therapeutics. 2020; 42(1):94-107.
MAT-XU-2503036 (v1.0) Date of Preparation: July 2025