- Article
- Source: Campus Sanofi
- 23 Jul 2025
Specific characteristics of Praluent® (alirocumab)


Pharmacological properties
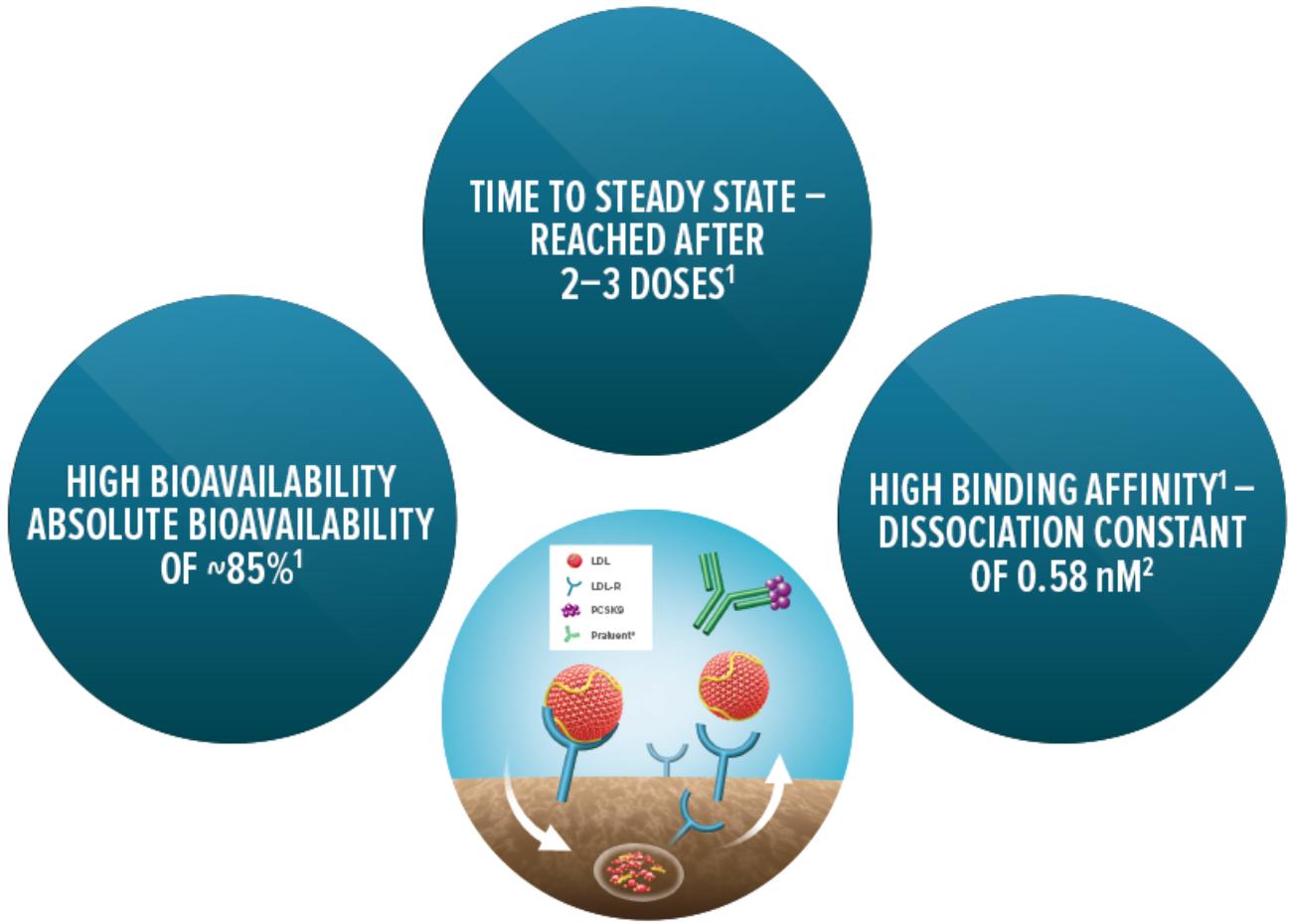
Figure © Sanofi.
Praluent®
Find more information on Indication, Administration and Mechanism of Action and watch videos about Praluent®.
.png)
References
- Praluent Summary of Product Characteristics. Available at https://www.medicines.org.uk/emc/product/8093/smpc. Accessed July 2025.
- Kuhnast S, et al. J Lipid Res 2014;55:2103–2112.
MAT-XU-2503027 (v1.0) Date of Preparation: July 2025