- Article
- Source: Campus Sanofi
- 5 Jan 2025
Nexviadyme®▼ (avalglucosidase alfa) evidence
Evidence overview
NEO1 (Phase 1)1
A multicentre, multinational, open-label, ascending dose study of Nexviadyme in ERT-experienced and -naïve patients with LOPD.
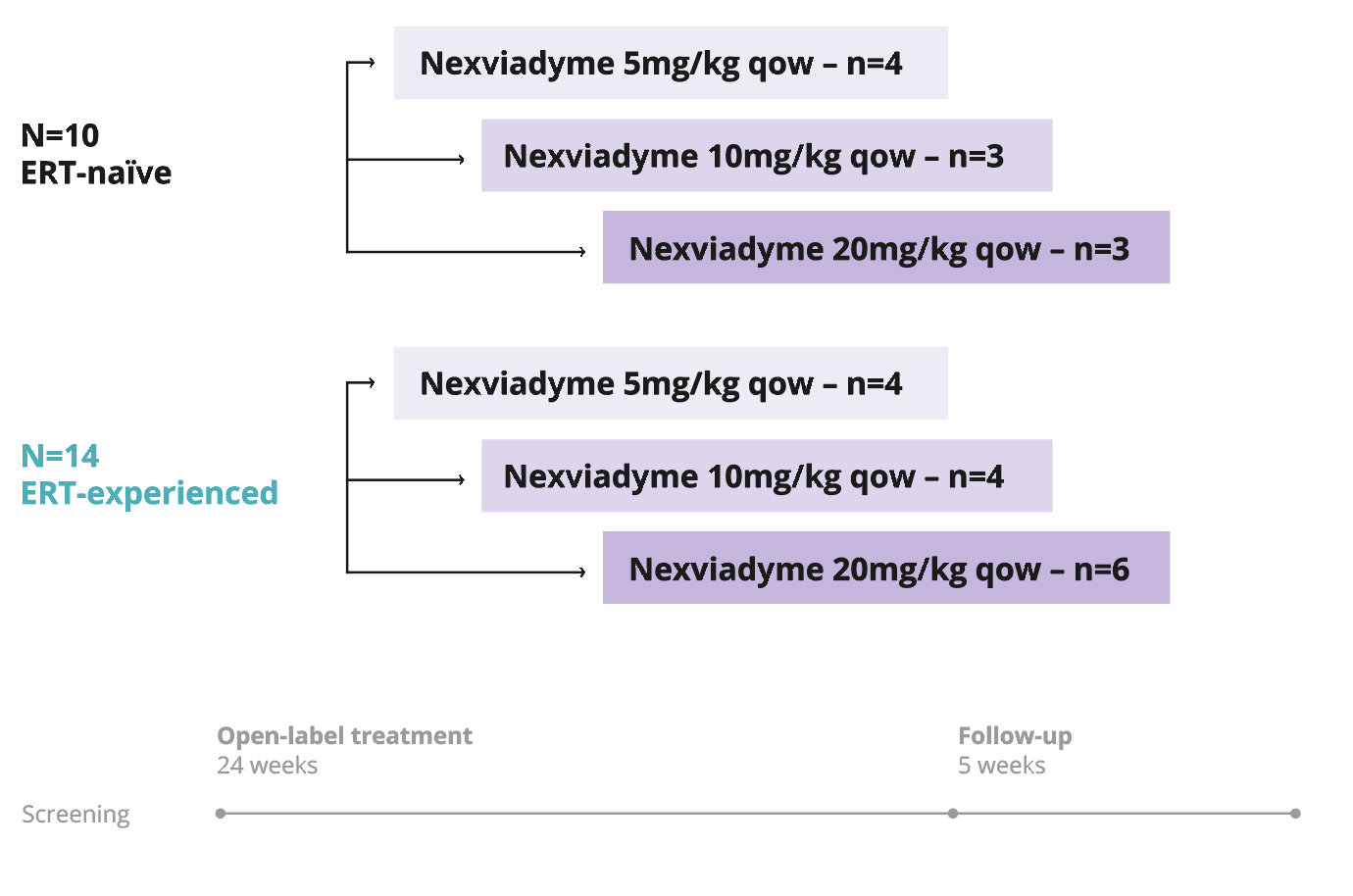
Primary
Safety, tolerability, pharmacokinetics, pharmacodynamics, and exploratory efficacy.
- ≥18 years old.
- Confirmed GAA enzyme deficiency and/or two confirmed GAA variants.
- Upright FVC ≥50% predicted.
- Able to walk ≥50 m without stopping or assistive device.
NEO-EXT (Phase 2)2
A multicentre, multinational, open-label study of Nexviadyme in ERT-experienced and -naïve patients with LOPD.
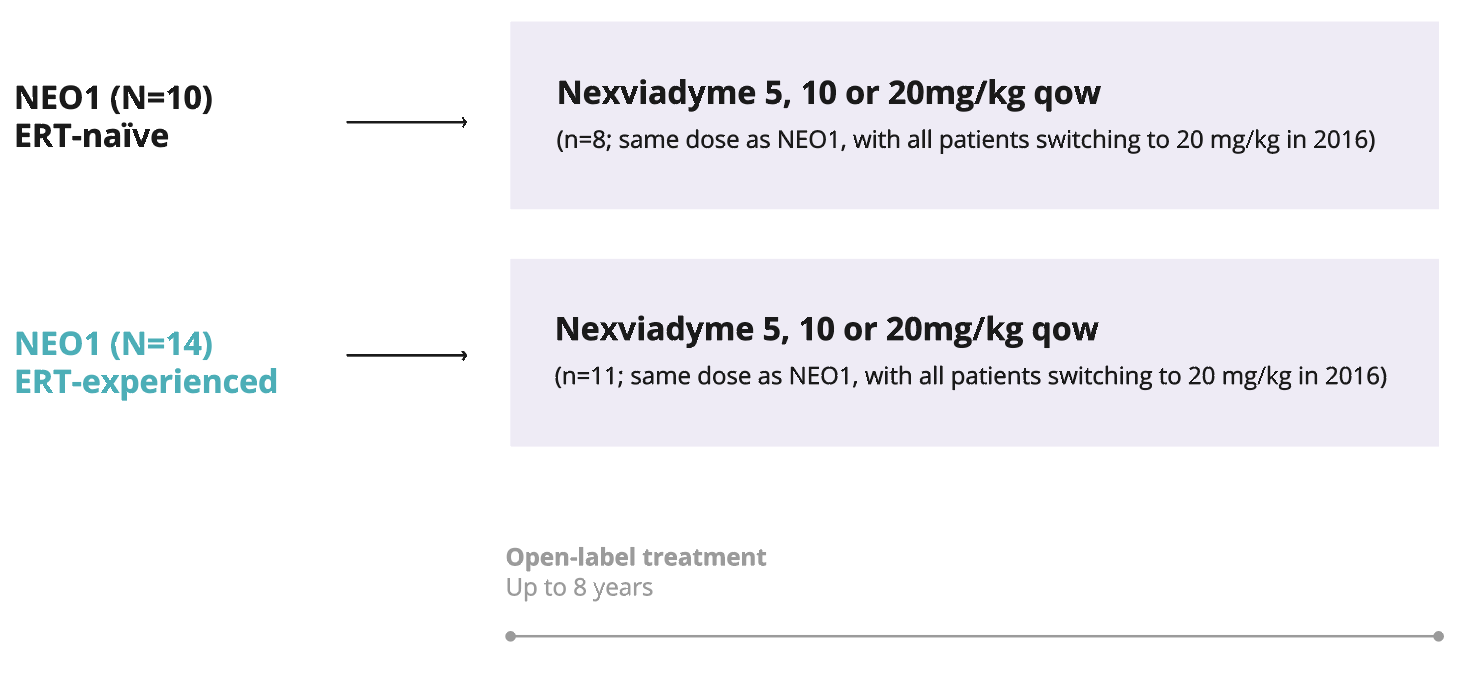
Primary
Long-term safety and pharmacokinetics.
Secondary
Long-term effect of Nexviadyme® on pharmacodynamic and exploratory efficacy variables, and the time-course of response.
-
Participated in NEO1 Phase 1 study.
NEO1 (N=14) ERT-experienced5
Long-term treatment effect of Nexviadyme on FVC and 6MWT has been shown for up to 8 years in an uncontrolled open-label study in patients with LOPD (NEO1/NEO-EXT).5 Each line represents an individual patient.
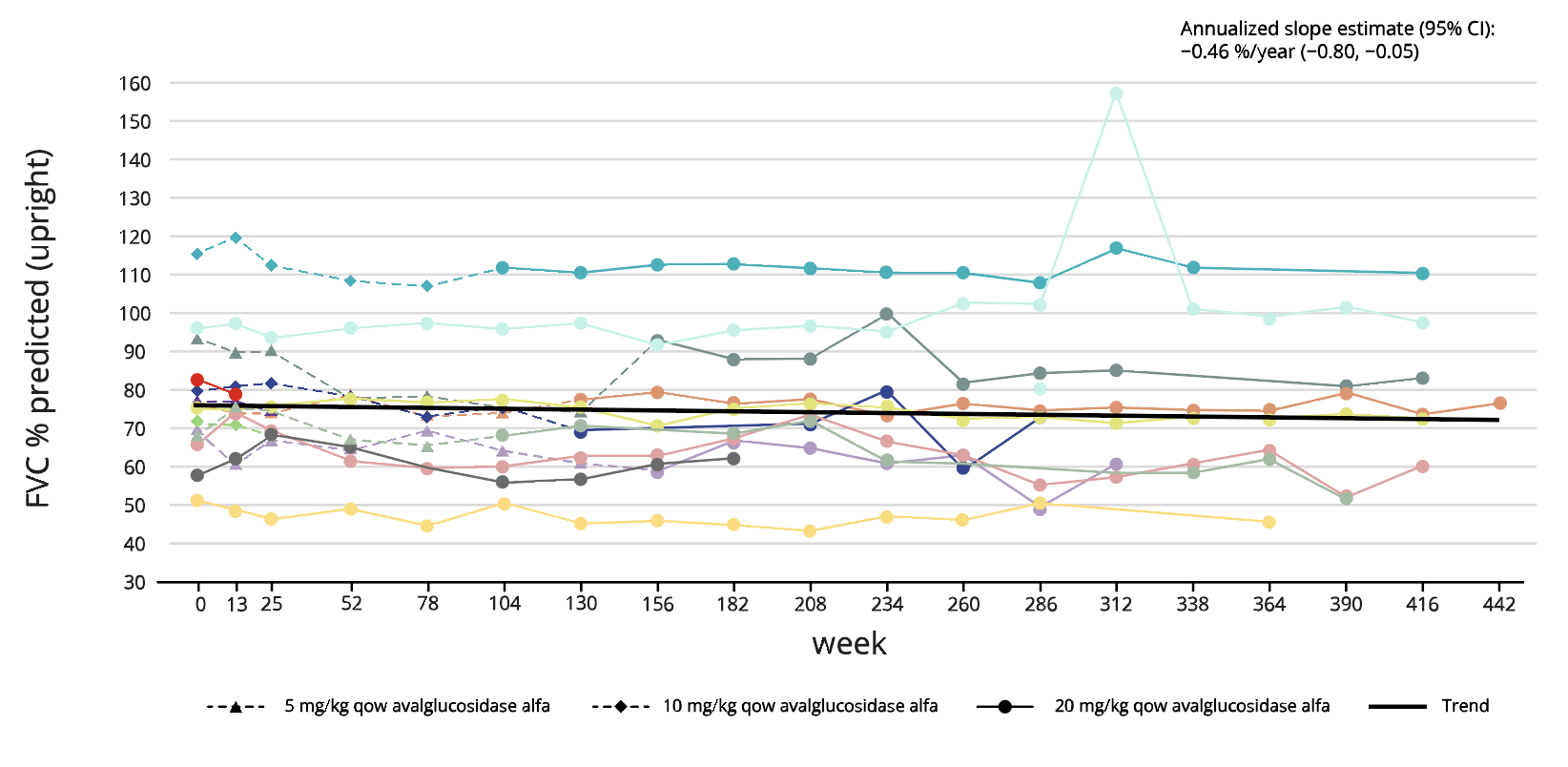
Adapted from Byrne BJ, et al. 2024.5
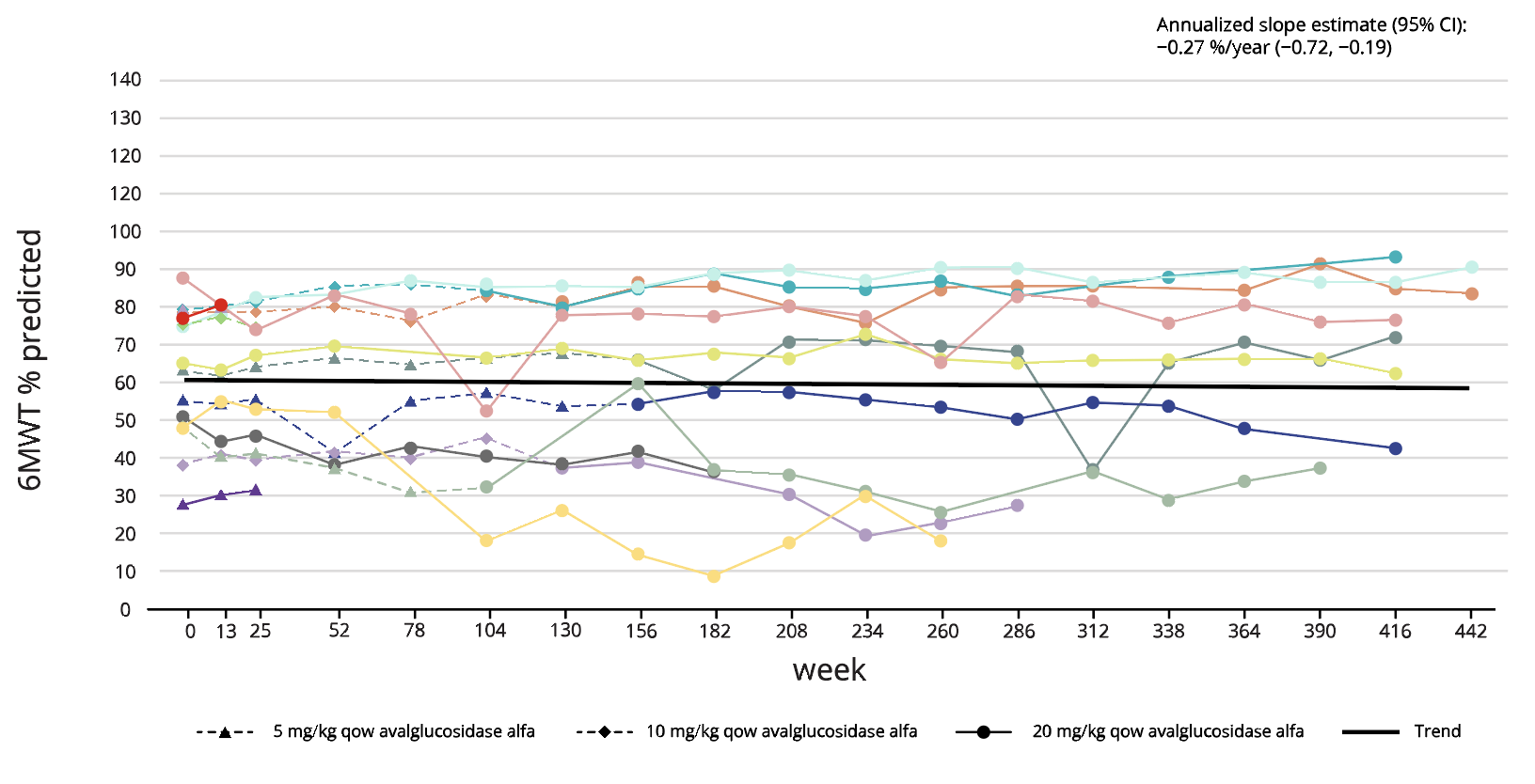
Adapted from Byrne BJ, et al. 2024.5
NEO1 (N=10) ERT-naïve5
Long-term treatment effect of Nexviadyme on FVC and 6MWT has been shown for up to 8 years in an uncontrolled open-label study in patients with LOPD (NEO1/NEO-EXT).5 Each line represents an individual patient.
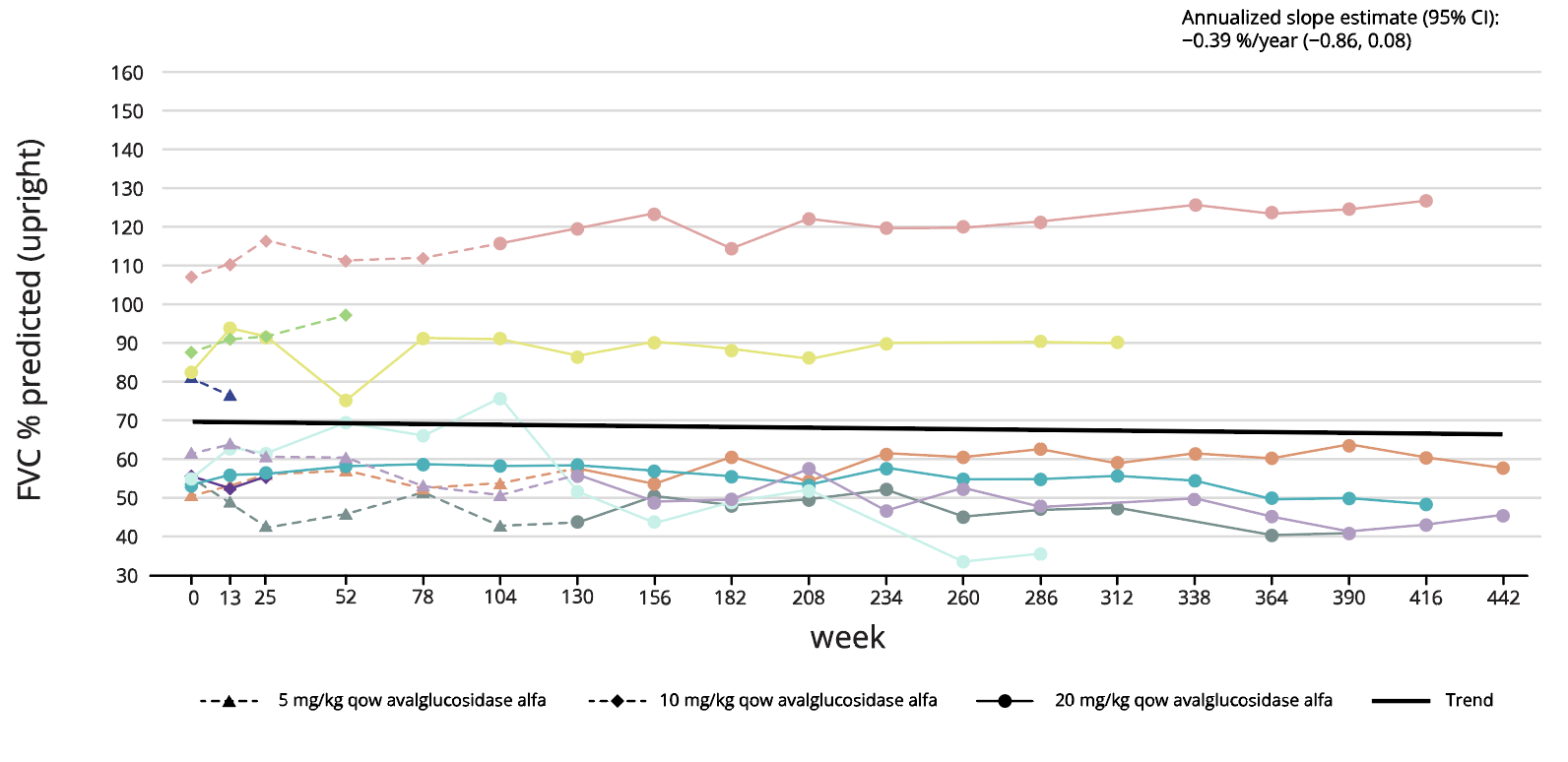
Adapted from Byrne BJ, et al. 2024.5
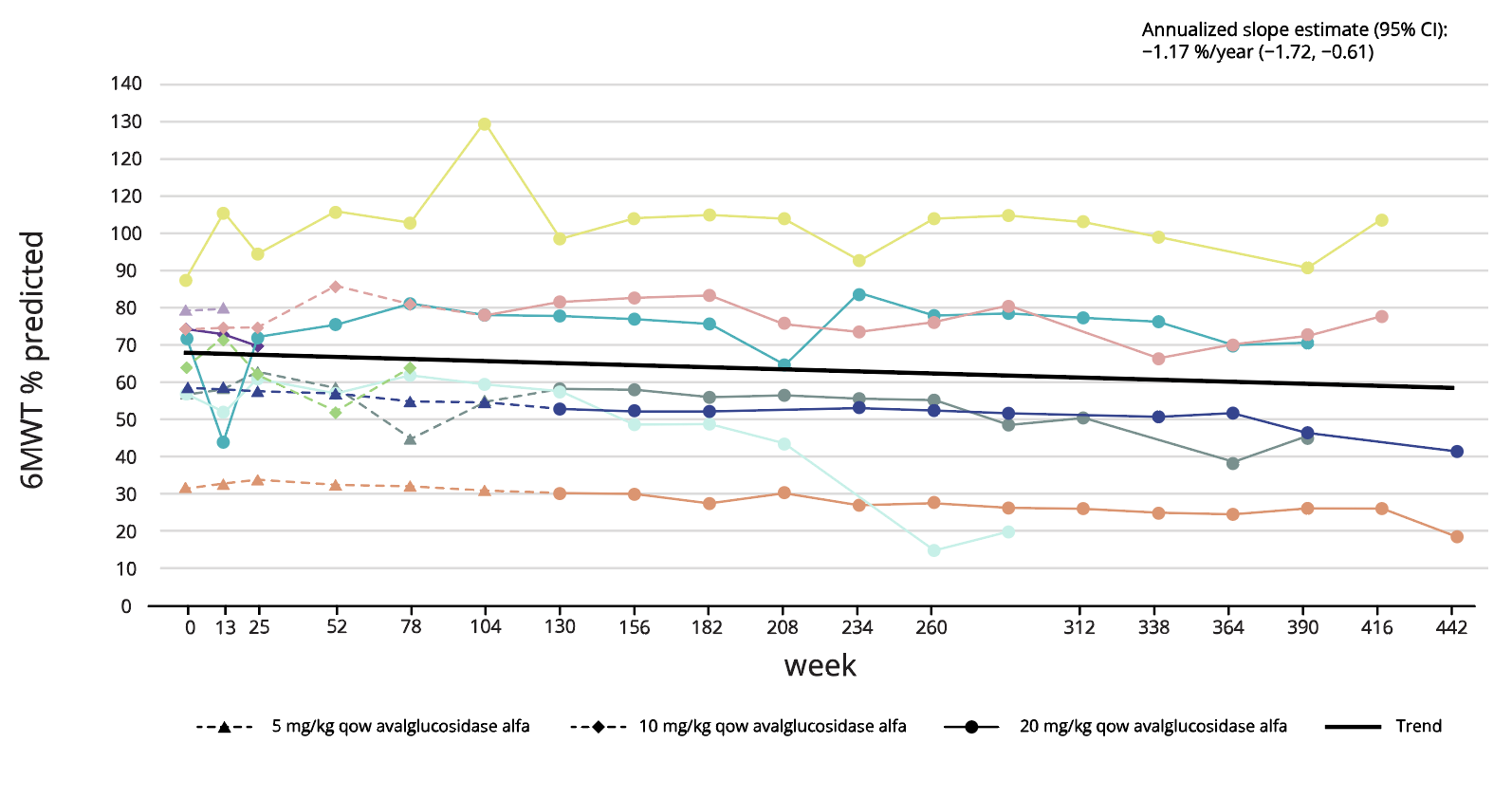
Adapted from Byrne BJ, et al. 2024.5
COMET trial (Phase 3)3
A multicentre, multinational, randomised, double-blinded study to compare the efficacy and safety of bi-weekly infusions of Nexviadyme and Myozyme in patients with LOPD.
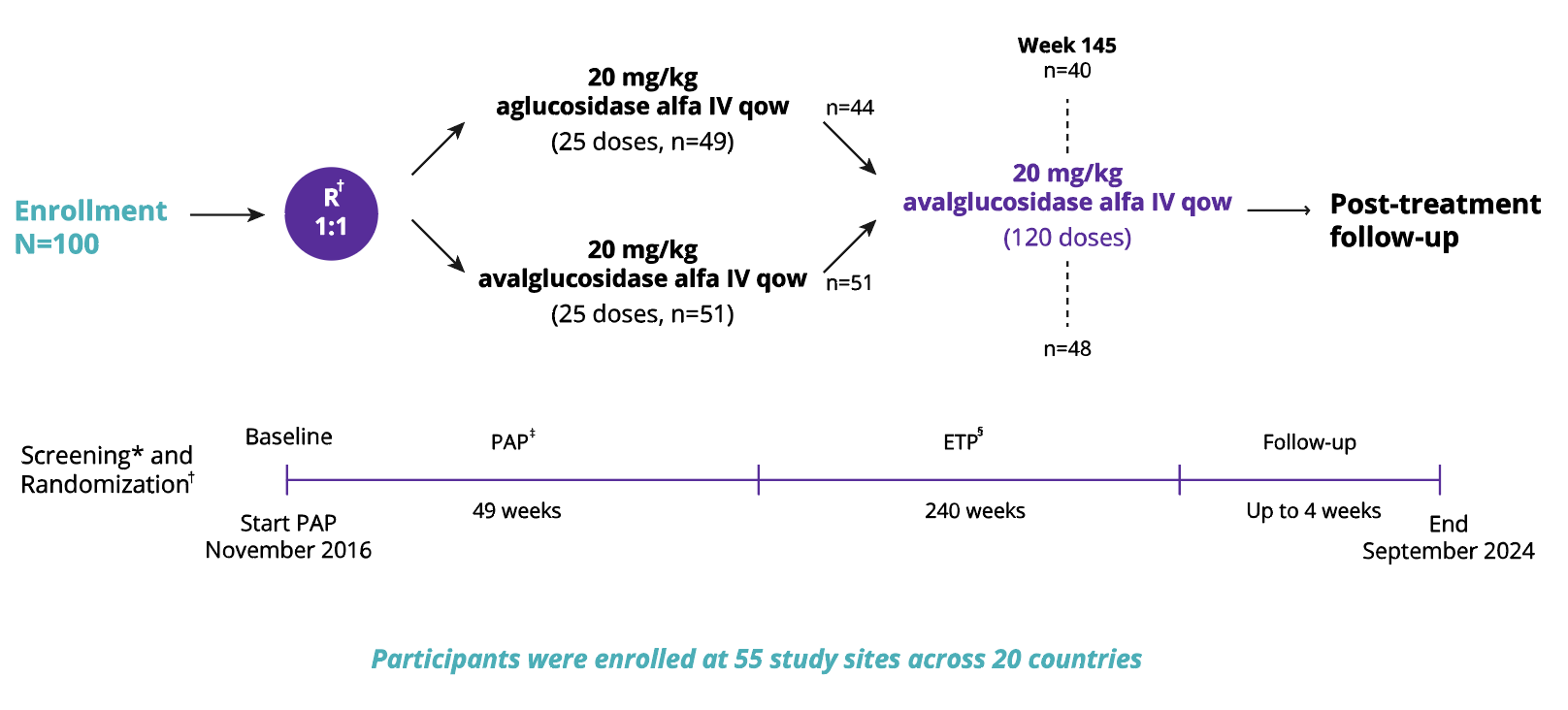
*Screening phase up to 14 days, may be extended to up to 8 weeks in pre-specified situations
†Randomization 1:1 ratio with stratification factors based on Baseline forced vital capacity, sex, age, and country (Japan or ex-Japan)
‡Study drug infusion, safety assessments, and efficacy evaluations
§All participants regardless of prior randomization
Primary
Change from baseline in FVC (% predicted) in the upright position.
Secondary
Change from baseline in 6MWT, MIP, MEP, HHD (lower extremity muscle strength), QMFT, SF-12, and safety.
-
≥3 years of age
-
Diagnosis of Pompe disease*
-
Naïve to treatment with alglucosidase alfa or any investigational therapy for Pompe disease
-
Able to successfully perform repeated FVC measurements of ≥30% & ≤85% predicted (upright)
-
Able to ambulate 40 meters (≈130 feet) without stopping and without an ambulation assistance device
FVC (% predicted), primary endpoint6
LS mean improvement of 1.40% with Nexviadyme and 1.18% in switch arm participants at week 145 vs baseline (statistical significance not reported).6
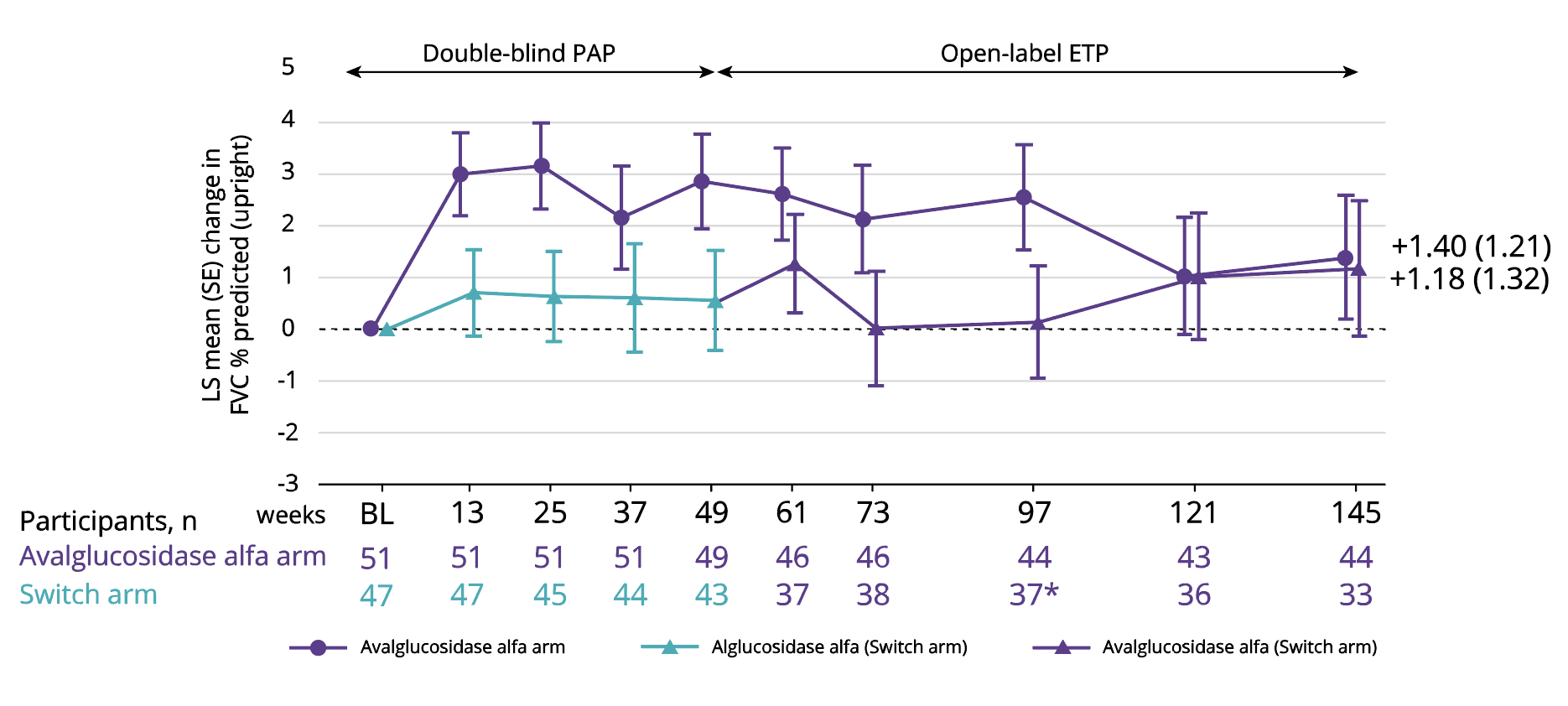
Adapted from Kishnani PS, et al. 2023.6
*1 participant’s FVC % predicted value at Week 97 was excluded due to a physiologically implausible change between Weeks 73 & 97 and 97 & 121.
6MWT, secondary endpoint6
LS mean improvement in 6MWT of 20.65 m with Nexviadyme and 0.29 m in switch arm participants at week 145 vs baseline (statistical significance not reported).6
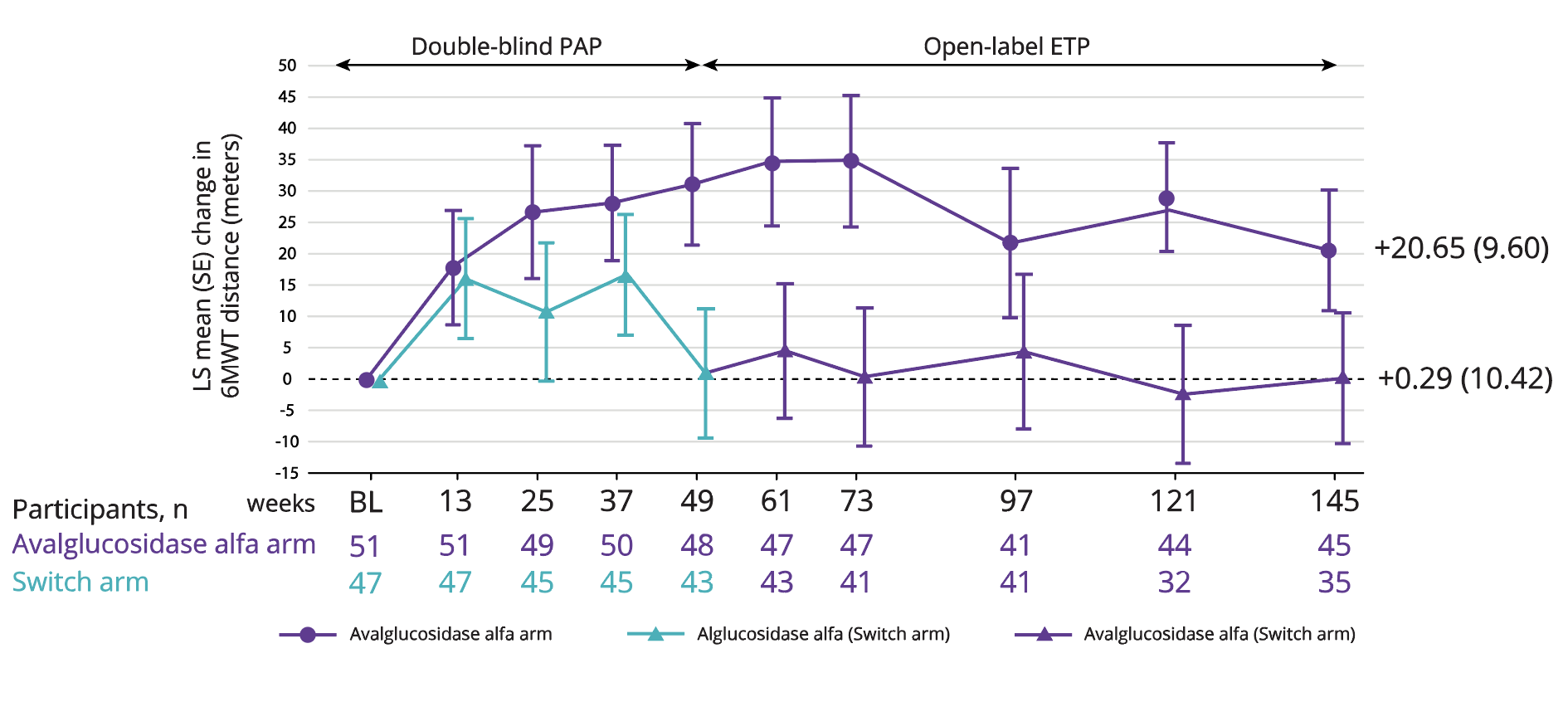
Adapted from Kishnani PS, et al. 2023.6
LS mean improvement in MIP of 6.04 with Nexviadyme and 7.53 in switch arm participants at week 145 vs baseline (statistical significance not reported).6
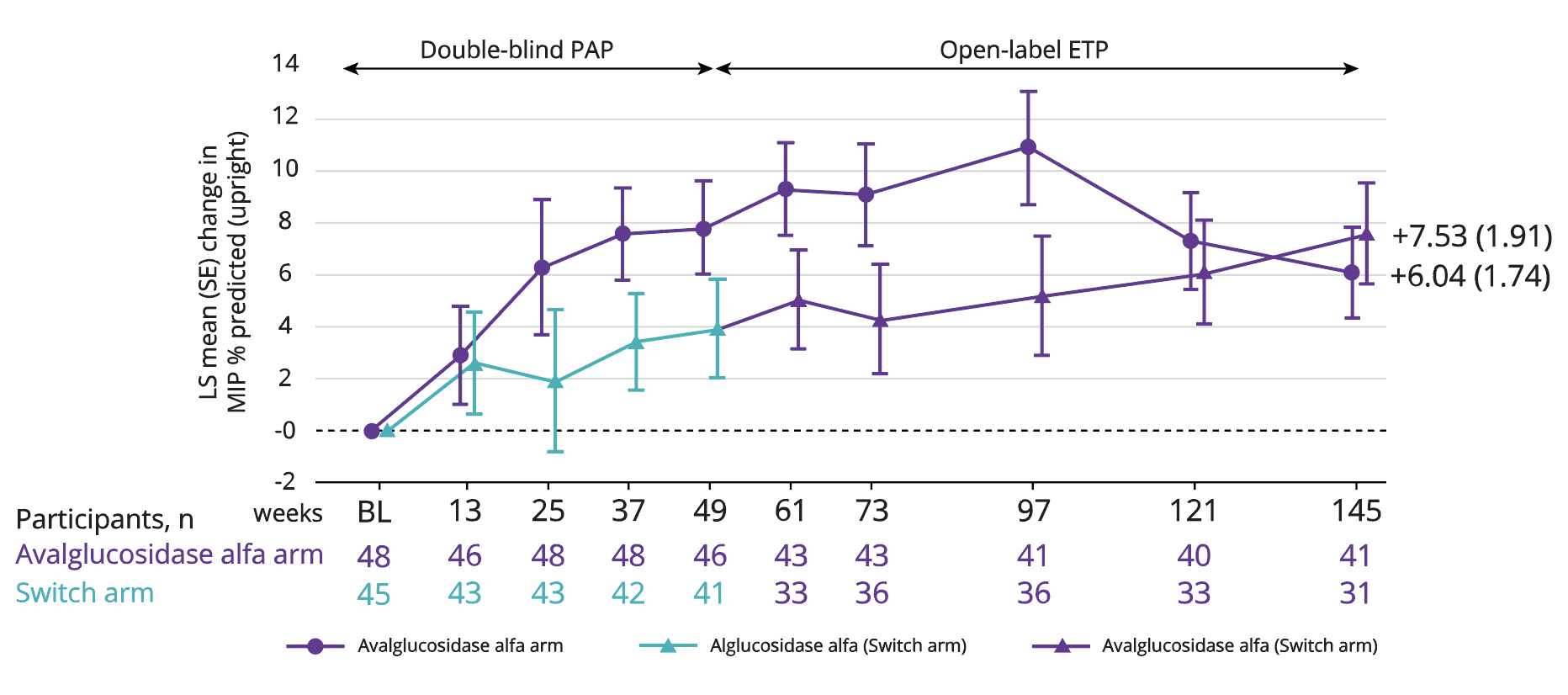
Adapted from Kishnani PS, et al. 2023.6
LS mean improvement in MEP of 16.68 with Nexviadyme and 14.70 in switch arm participants at week 145 vs baseline (statistical significance not reported).6
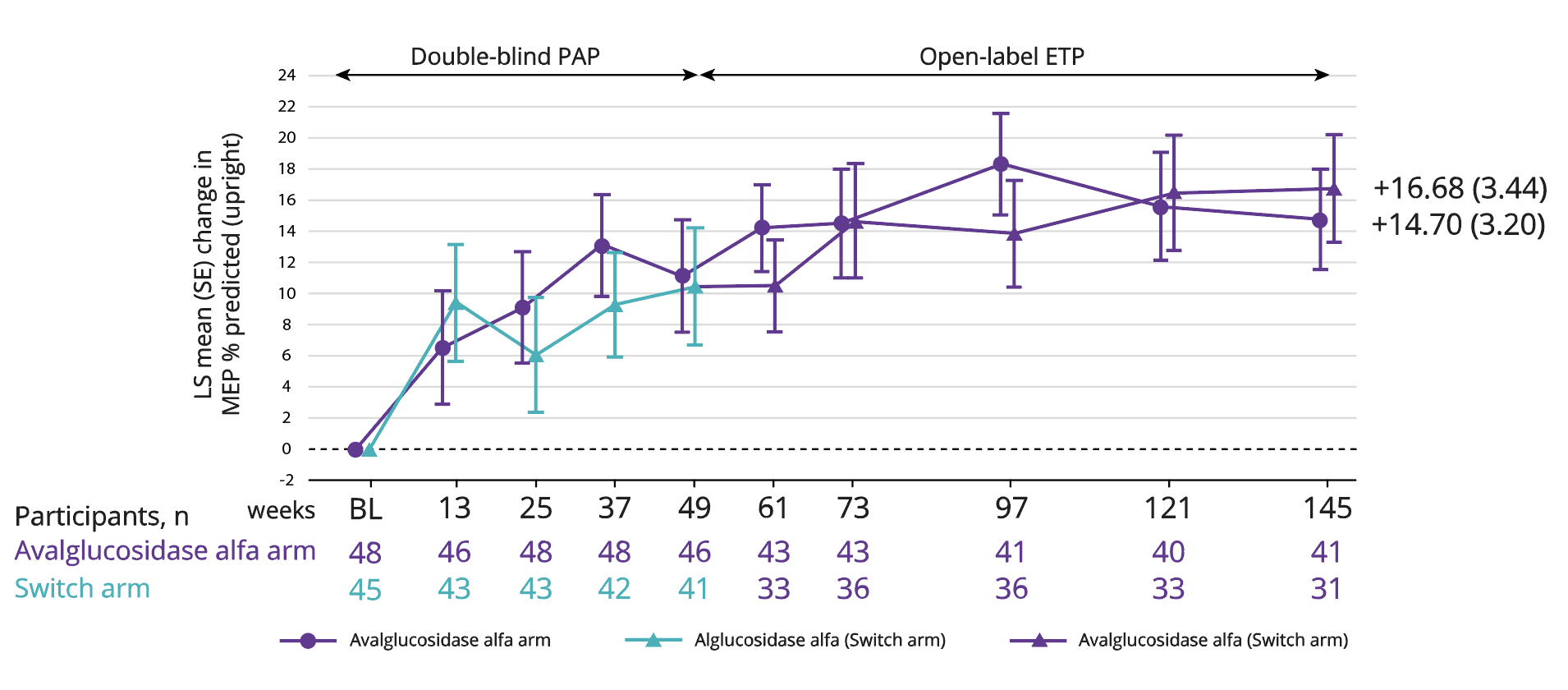
Adapted from Kishnani PS, et al. 2023.6
†4 participants (2 in each group) were excluded from the MIP and MEP % predicted analyses due to implausible values at Baseline.
LS mean improvement in HHD (lower extremity strength) of 177.22 with Nexviadyme and 154.90 in switch arm participants at week 145 vs baseline (statistical significance not reported).6
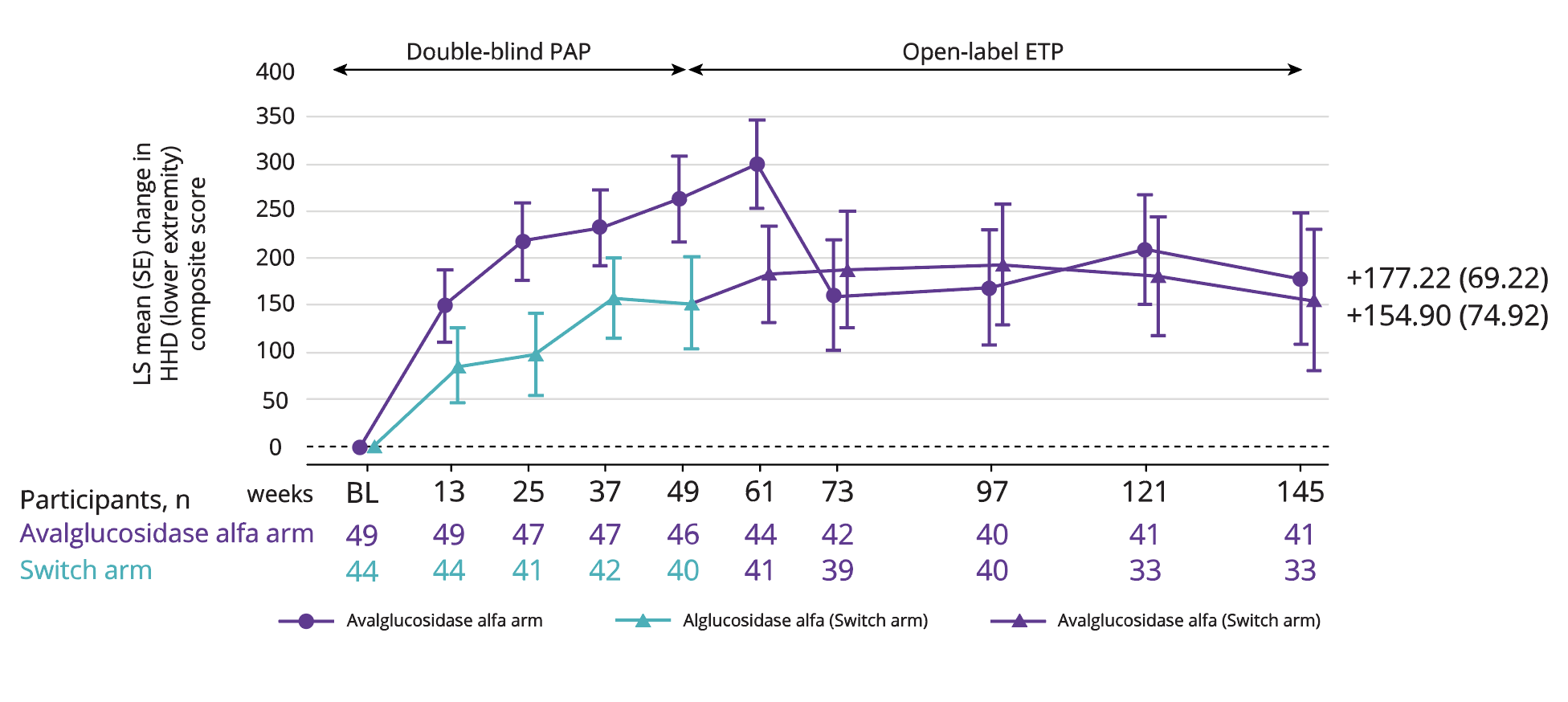
Adapted from Kishnani PS, et al. 2023.6
LS mean improvement in QMFT (motor function) of 4.27 with Nexviadyme and 1.93 in switch arm participants at week 145 vs baseline (statistical significance not reported).6
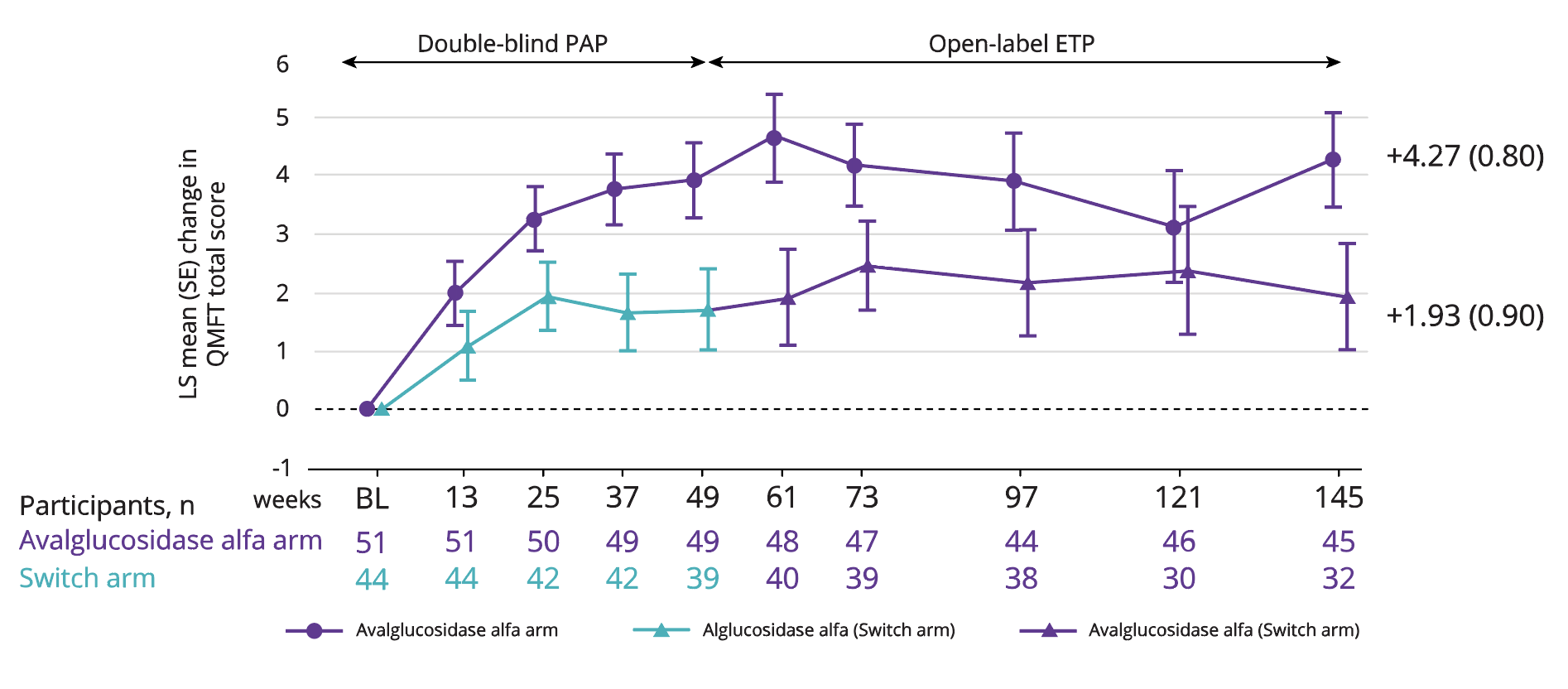
Adapted from Kishnani PS, et al. 2023.6
SF-12 MCS score was stabilized from Baseline for both the avalglucosidase alfa and switch arms (mean LS [SE] change from Baseline: −0.04 [1.28] and −0.79 [1.15], respectively).6
SF-12 PCS score improved from Baseline in both the avalglucosidase alfa and switch arms (mean LS [SE] change from Baseline: +4.07 [0.88] and +3.83 [0.98], respectively).6
Mean reduction in Hex4 levels of 53.35% with Nexviadyme and 48.04% in switch arm participants at week 145 vs baseline (statistical significance not reported).6
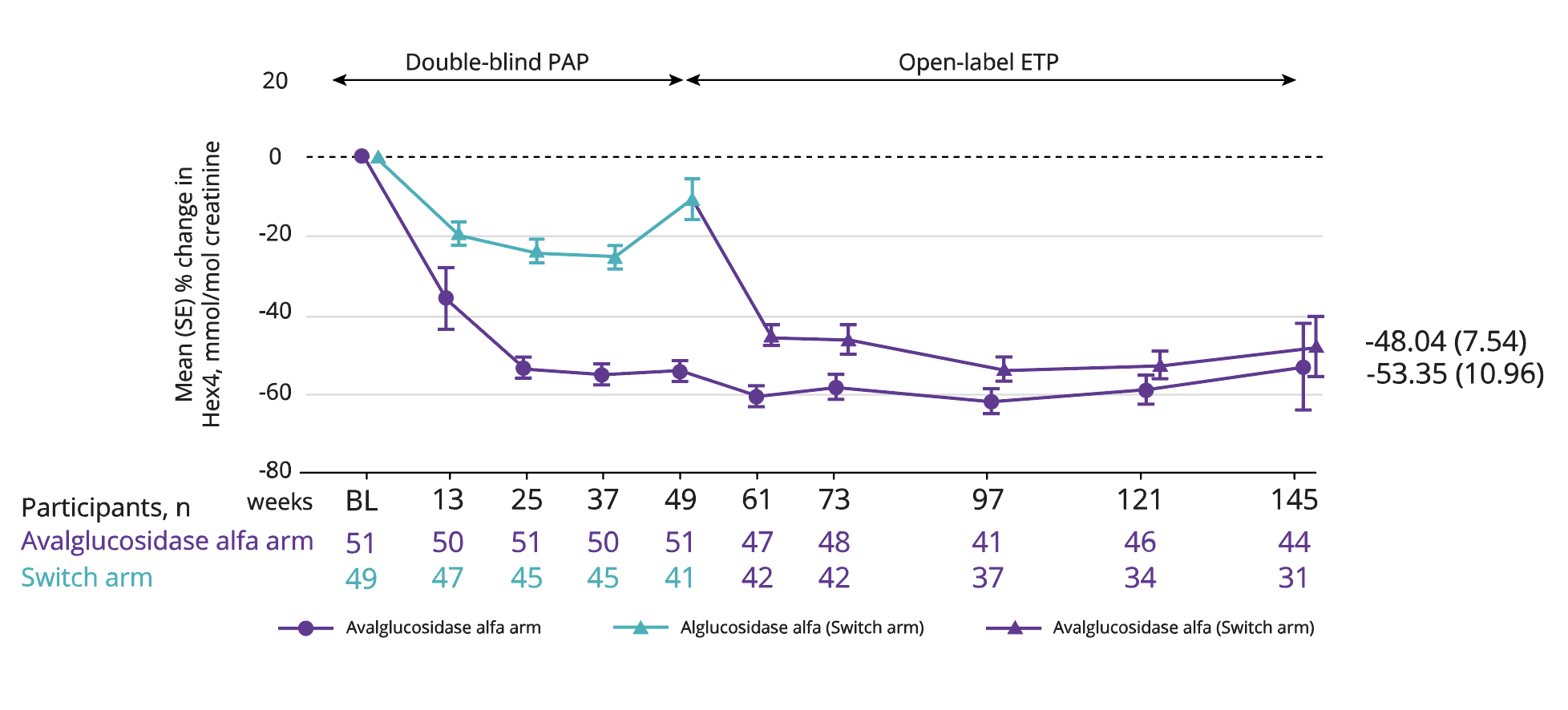
Adapted from Kishnani PS, et al. 2023.6
Mini-COMET trial4
Of 22 included patients with IOPD (1 year of age of older, declining or with sub-optimal response on Myozyme), 16 received Nexviadyme and showed improvement or stabilisation based on secondary outcome measures of preliminary efficacy. The primary outcome measure of Mini-COMET was safety.
This was shown in Mini-COMET across cardiac function, motor function and mobility.8
Cardiac function
Assessed by ECHO-LVM
z score
Motor function
Assessed by the GMFM-88
and QMFT
Mobility
Assessed by the Pompe-
PEDI
Patients showed stabilisation or improvement in the mobility domain of the Pompe-PEDI.8
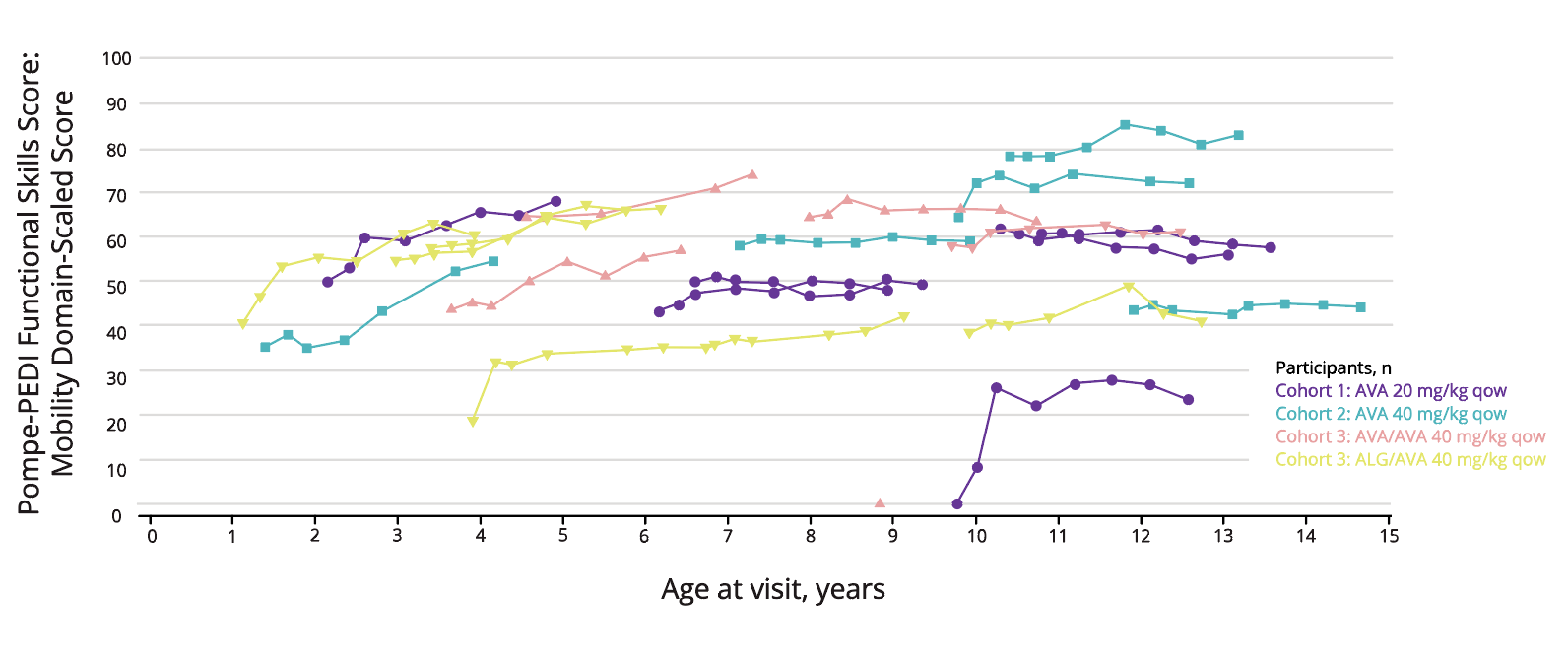
Adapted fron Kronn D, et al. 2024.8
Patients are represented by separate lines, with the first dot representing the age at inclusion in the trial and last dot at conclusion for this trial period.
The Pompe-PEDI is based on caregiver reports.
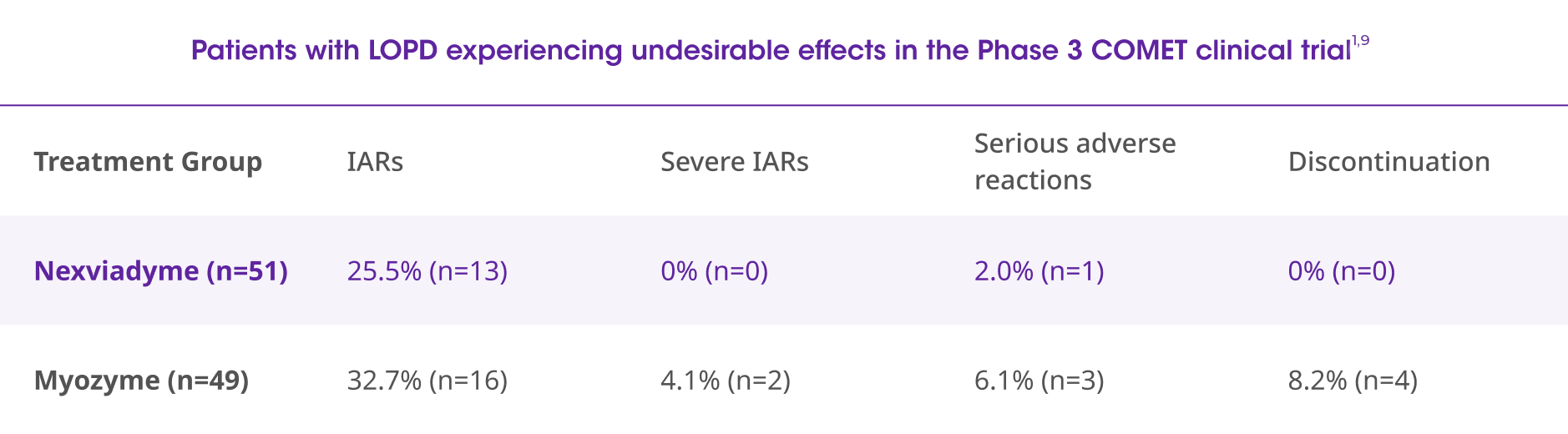
Safety
In the COMET trial, there were fewer numerical treatment-related SAEs and IARs with Nexviadyme than with Myozyme. During the 49-week primary analysis period, no patients withdrew from the Nexviadyme arm.3
Learn more about Nexviadyme®

Nexviadyme® safety profile
Learn more about the safety and tolerability profile for Nexviadyme®.

Treatment with Nexviadyme®
Read more about treatment with Nexviadyme® for patients living with Pompe disease.

Nexviadyme® safety profile
Learn more about the safety and tolerability profile for Nexviadyme®.

Treatment with Nexviadyme®
Read more about treatment with Nexviadyme® for patients living with Pompe disease.
Do you have questions or need support? We are here to help you.
6MWT, 6-minute walk test; ALG, alglucosidase alfa; AVA, avalglucosidase alfa; BL, Baseline; CI, confidence interval; CK, creatinine kinase; ECHO, echocardiography; ERT, enzyme replacement therapy; ETP, extended treatment period; FVC, forced vital capacity; GAA, acid alpha-glucosidase; GMFCS-E&R, Gross Motor Function Classification System – Expanded and Revised; GMFM-88, Gross Motor Function Measure-88; Hex4, hexose tetrasaccharide; HHD, hand-held dynamometry; HRQoL, health-related quality of life; IARs, infusion-associated reactions; IOPD, infantile onset Pompe disease; IV, intravenous; LOPD, late-onset Pompe disease; LS, least-squares; LVM, left ventricular mass; MCS, mental component summary; MEP, maximum expiratory pressure; MIP, maximum inspiratory pressure; OLE, open-label extension; PAP, primary analysis period; PCS, physical component summary; Pompe-PEDI, Pompe-specific Paediatric Evaluation of Disability Inventory; QMFT, quick motor function test; qow, every other week; qw, every week; R, randomization; SAEs, serious adverse events; SD, standard deviation; SE, standard error; SF-12, 12-Item Short Form Survey.
References
-
Pena LDM, et al. Safety, tolerability, pharmacokinetics, pharmacodynamics, and exploratory efficacy of the novel enzyme replacement therapy avalalucosidase alfa (neoGAA) in treatment-naive and alglucosidase alfa-treated patients with late-onset Pompe disease: A phase 1, open-label, multicenter, multinational, ascending dose study. Neuromuscul Disord. 2019 Mar;29(3):167-186.
-
Dimachkie MM, et al. Long-term Safety and Efficacy of Avalglucosidase Alfa in Patients With Late-Onset Pompe Disease. Neurology. 2022 May 26;99(5):e536-48.
-
Diaz-Manera J, et al. Safety and efficacy of avalglucosidase alfa versus alglucosidase alfa in patients with late-onset Pompe disease (COMET): a phase 3, randomised, multicentre trial. Lancet Neural. 2021 Dec;20(12):1012-1026.
-
Kishnani PS, et al. Safety and efficacy of avalglucosidase alfa in individuals with infantile-onset Pompe disease enrolled in the phase 2, open-label Mini COMET study: The 6-month primary analysis report. Genet Med. 2023 Feb;25(2):100328. Epub 2022 Dec 21.
-
Byrne BJ, et al. NEO1/NEO-EXT Studies: Long-Term Muscle Quantitative Magnetic Resonance Imaging and Functional Efficacy in Adults With Late-Onset Pompe Disease (LOPD) on Avalglucosidase Alfa Treatment. Poster presented at the 20th Annual WORLDSymposium 2024, February 4 -9, 2024, San Diego, CA, USA.
-
Kishnani PS, et al. Efficacy and Safety of Avalglucosidase Alfa in Participants With Late-Onset Pompe Disease After 145 Weeks' Treatment During the COMET Trial. ePoster presented in Orlando, Florida, USA, February 22 -26, 2023.
-
Kishnani PS, et al. COMET Investigator Group. Efficacy and Safety of Avalglucosidase Alfa in Patients With Late-Onset Pompe Disease After 97 Weeks: A Phase 3 Randomized Clinical Trial. JAMA Neurol. 2023 Jun 1;80(6):558-567. doi: 10.1001/jamaneurol.2023.0552.
-
Kronn D, et al. Mini-COMET Study: Safety and Efficacy Data After Avalglucosidase Alfa Dosing for ≥145 Weeks in Participants With Infantile-Onset Pompe Disease (IOPD) Who Had Demonstrated Clinical Decline or Sub-Optimal Response Whilst Receiving Alglucosidase Alfa. Encore poster presented at the 20th Annual WORLDSymposium 2024, February 4 -9, 2024, San Diego, CA, USA.
-
Sanofi. Nexviadyme (avalglucosidase alfa). Summary of Product Characteristics. 2024. Available at: https://www.medicines.org.uk/emc/product/14562/smpc#gref.
MAT-XU-2400914 (v3.0) Date of preparation: December 2025