Dosing and administration instructions
Administration
TZIELD is administered by intravenous infusion over a minimum of 30 minutes, using body surface area-based (BSA) dosing1
Dosage form and strength
2 mg/2 mL (1 mg/mL), clear and colourless solution in a single-dose vial1
No same-day dosing
Do NOT administer two doses on the same day1
If an infusion is missed
Resume by administering all remaining doses on consecutive days to complete the 14-day course1
Recommended dosing schedule for TZIELD
The recommended TZIELD dosage for adults and paediatric patients aged 8 years and older uses BSA-based dosing and is administered according to the following regimen:*1
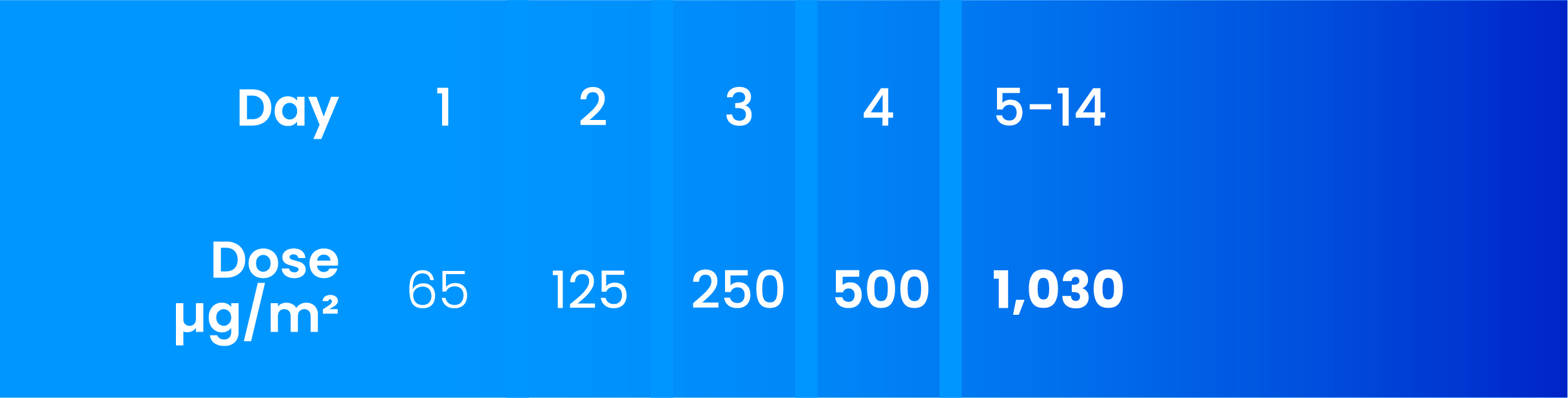
Premedicate prior to TZIELD infusion for the first five days of dosing with a non-steroidal anti-inflammatory drug (NSAID) or paracetamol, an antihistamine, and/or an antiemetic. Administer additional doses of premedication if needed.1
Refer to section 6.3, section 6.4, and section 6.6 of the Summary of Product Characteristics (SmPC) for information on storage and preparation for intravenous administration of TZIELD.
How to calculate BSA dosing with the Mosteller formula2
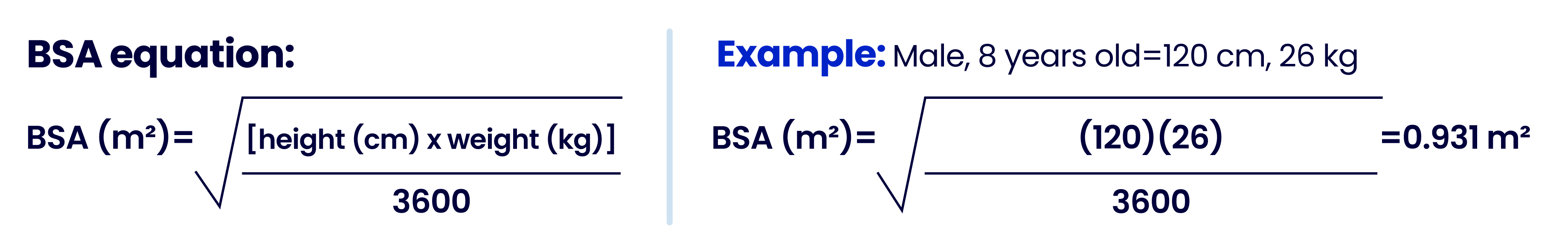
- When calculating BSA, round to the 100th using standard rounding rules (example: 0.93 m2)
- Based on BSA dosing requirements, two vials maybe needed for some individuals (BSA >1.94 m2) for days 5–141
BSA Calculation
Dosing Regimen
BSA: m²
Patient selection
Select adult and paediatric patients 8 years of age and older for TZIELD treatment who have a diagnosis of Stage 2 T1D1
- Confirm Stage 2 T1D by documenting:
- At least two positive pancreatic islet cell autoantibodies (AAbs)
- Dysglycaemia without overt hyperglycaemia
- Ensure the clinical history of the patient does not suggest Type 2 diabetes (T2D)
Laboratory evaluation and vaccinations prior to treatment
Prior to initiation of TZIELD, there are a number of laboratory evaluations required to ensure that TZIELD is suitable for patients. All age-appropriate vaccinations must also be administered before treatment1
- Prior to initiating TZIELD, obtain a complete blood count and liver enzyme tests
- Use of TZIELD is not recommended in patients with:
- Lymphocyte count <109 lymphocytes/L
- Haemoglobin <100 g/L
- Platelet count <150 x 109 platelets/L
- Absolute neutrophil count <1.0 x 109 neutrophils/L in those of African descent and <1.5 x 109 neutrophils/L in all other groups
- Elevated alanine aminotransferase (ALT) or aspartate aminotransferase (AST) >2 x upper limit of normal (ULN)
- Laboratory or clinical evidence of acute infection with Epstein-Barr virus (EBV) or cytomegalovirus (CMV)
- Active serious infection or chronic active infection other than localised skin infections
- Administer all age-appropriate vaccinations prior to starting TZIELD:
- Administer live-attenuated (live) vaccines at least 8 weeks prior to treatment
- Administer inactivated (killed) vaccines or messenger ribonucleic acid (mRNA) vaccines at least 2 weeks prior to treatment
Get in Touch with Us
Questions? Leave your details and we'll reach out to you at your preferred time.
Get in touchINDICATION: TZIELD is indicated to delay the onset of Stage 3 T1D in adult and paediatric patients 8 years of age and older with Stage 2 T1D.1
*The dosing schedule in TN-10 was different to the recommended dosing schedule in the SmPC.1
AAbs, autoantibodies; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BSA, body surface area; CMV, cytomegalovirus; EBV, Epstein-Barr virus; mRNA, messenger ribonucleic acid; NSAID, non-steroidal anti-inflammatory drug; SmPC, Summary of Product Characteristics; T2D, Type 2 diabetes.
- TZIELD® (teplizumab) UK Summary of Product Characteristics. 2025.
- Mosteller RD. N Engl J Med. 1987; 317(17): 1098.
MAT-XU-2500768 (v1.0) | November 2025
