INDICATION: TZIELD is indicated to delay the onset of Stage 3 Type 1 diabetes (T1D) in adult and paediatric patients 8 years of age and older with Stage 2 T1D.1
TN-10 study design
A phase 2, randomised, double-blind, event-driven, placebo-controlled study in 76 patients, 8–49 years of age with Stage 2 T1D and who had a relative diagnosed with Stage 3 T1D.*1,2

TZIELD dosing in the TN-10 trial is different to that recommended in the approved Summary of Product Characteristics (SmPC).†1,2 However, total drug exposure was comparable to that achieved with the recommended total TZIELD dosage.1
Primary efficacy endpoint:
Time from randomisation to diagnosis of Stage 3 clinical T1D.‡1
Efficacy from the TN-10 primary analysis
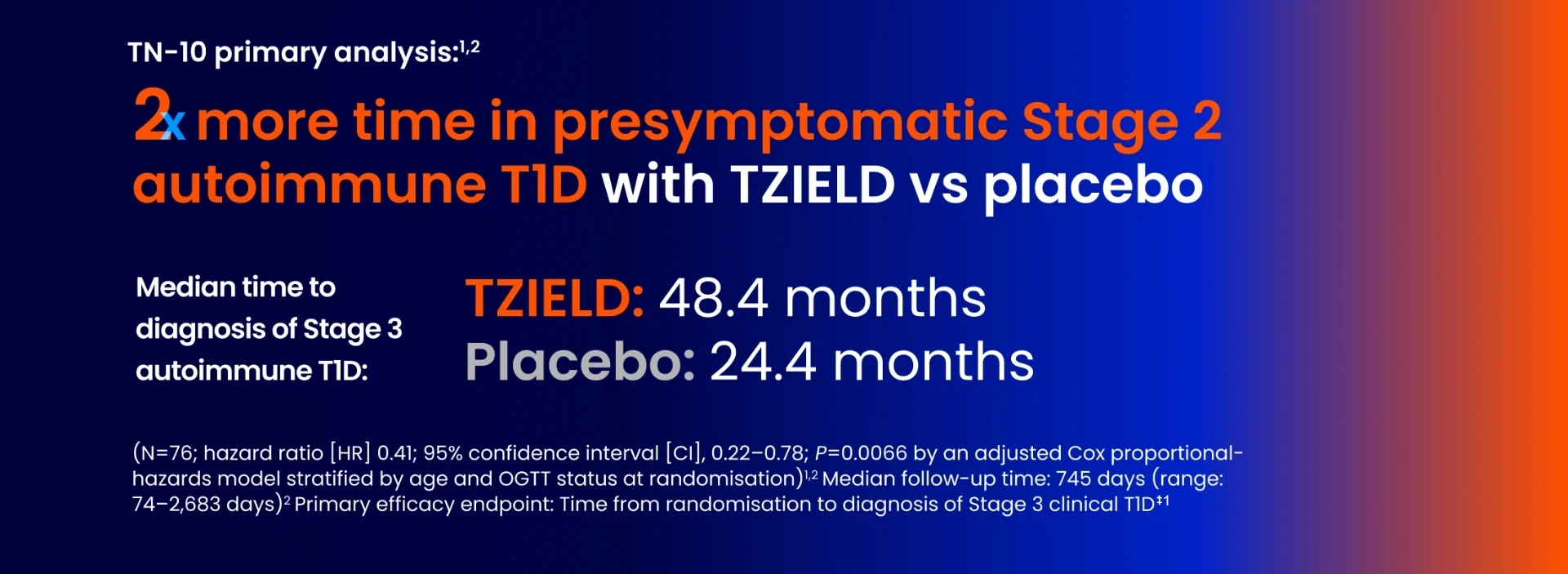
In TN-10, Stage 3 T1D was diagnosed in 20 (45%) patients treated with TZIELD and 23 (72%) patients treated with placebo.1
Kaplan-Meier curve of time to diagnosis of Stage 3 T1D1
- HR 0.41; 95% CI, 0.22–0.78; P=0.0066 by an adjusted Cox proportional-hazards model stratified by age and OGTT status at randomisation1,2
- Tick marks indicate censored data
- At the end of the trial, 25 TZIELD-treated patients did not have a Stage 3 autoimmune T1D diagnosis vs 9 with placebo2
Adapted from TZIELD (teplizumab) UK SmPC. 2025.1
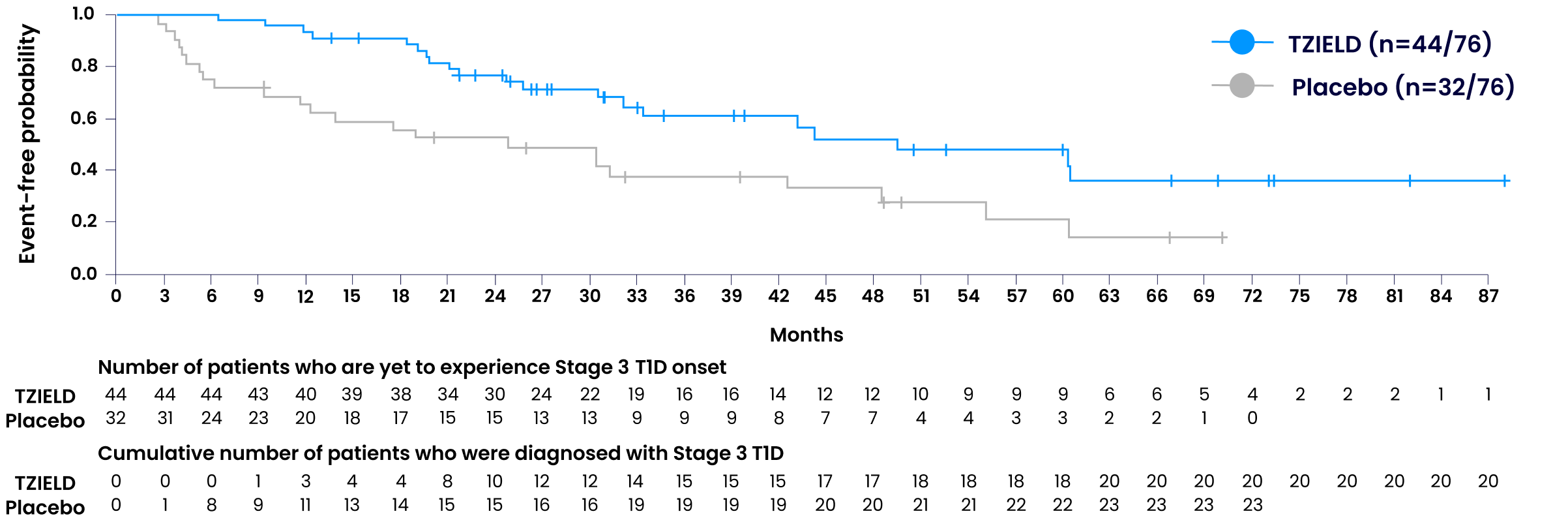
Data beyond 5 years3
In an extended analysis of the TN-10 study, 38.6% of TZIELD-treated patients (n=17/44) remained in presymptomatic Stage 2 autoimmune T1D for 5+ years vs 18.8% of placebo-treated patients (n=6/32).§3
Extended analysis limitations: The TN-10 study was relatively small at the start of the study, and patient numbers decreased throughout the follow-up. Therefore, definitive conclusions cannot be derived from these data.3
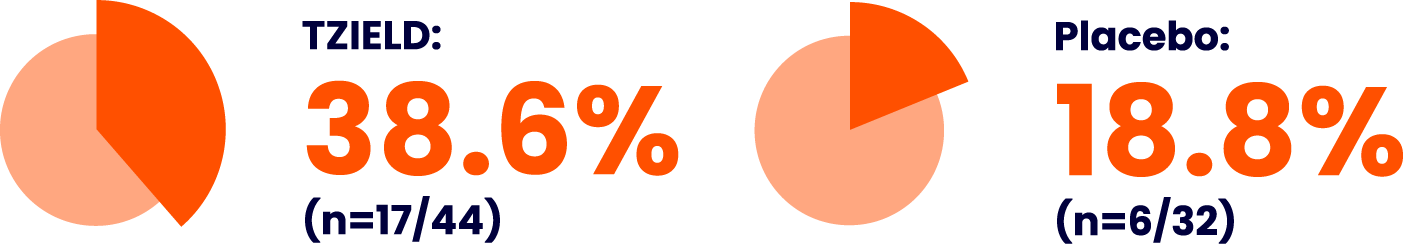
The safety profile of TZIELD has been evaluated in a pool of adult and paediatric patients across five controlled clinical studies, four in Stage 3 T1D (unapproved population) and one in Stage 2 T1D||1
Lymphopenia, leukopenia, neutropenia, decreased blood bicarbonate, and rash were the most frequently reported adverse reactions, which occurred at a higher frequency in the TZIELD group compared to the control group.||1 Please see the table below for further adverse reactions.
No drug interaction studies have been performed.1
Adverse reactions occurring in ≥5% of patients in the pooled safety analysis of clinical studies1
Adapted from TZIELD (teplizumab) UK SmPC. 2025.1
Serious adverse reactions have been reported with greater frequency in TZIELD-treated patients vs placebo-treated patients in TN-101
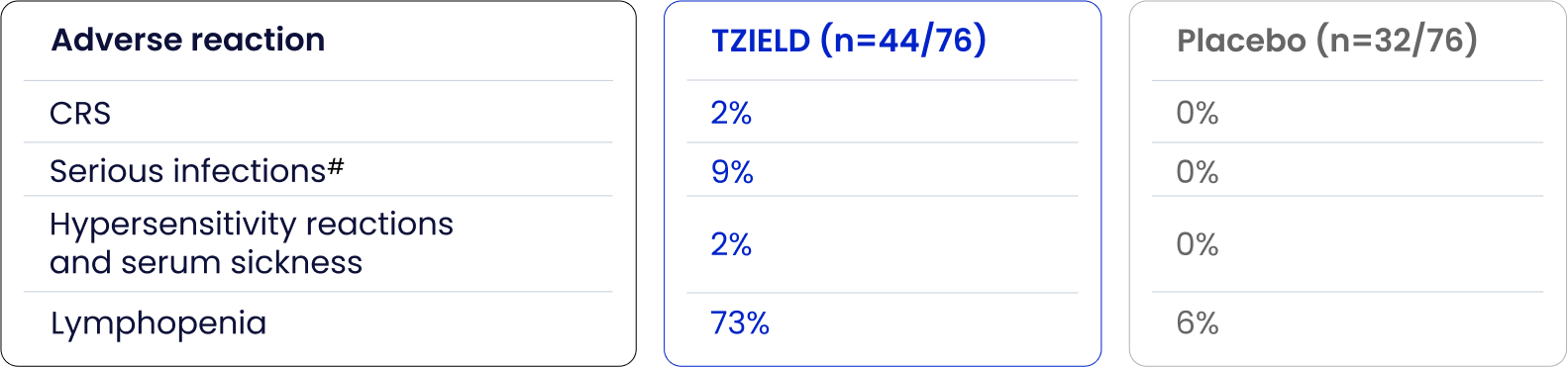
Adapted from TZIELD (teplizumab) UK SmPC. 2025.1
Refer to section 4.8 of the SmPC for more information regarding these and other adverse reactions.
Special warnings and precautions for use
- CRS manifestations in patients treated with TZIELD included fever, nausea, fatigue, headache, myalgia, arthralgia, increased ALT, increased AST, and increased total bilirubin, and typically occurred during the first 5 days of treatment1
- In clinical trials, CRS was reported in 6% of patients treated with TZIELD vs 1% of patients in the control group during the treatment period and through 28 days after the last study drug administration1
- 13% of these CRS cases were serious adverse reactions1
- Premedicate with antipyretics, antihistamines, and/or antiemetics prior to TZIELD treatment1
- Monitor liver enzymes and bilirubin during treatment. Discontinue TZIELD treatment in patients who develop elevated ALT or AST more than 5 times the upper limit of normal (ULN) or bilirubin more than 3 times the ULN1
- Treat symptoms of CRS with antipyretics, antihistamines, and/or antiemetics. If severe CRS develops, consider temporarily pausing dosing for 1–2 days (and administer the remaining doses to complete the full 14-day course on consecutive days) or discontinuing treatment1
TN-10 Week 6: Average lymphocyte counts return to baseline2

- Average lymphocyte count nadir occurred at Day 5 of treatment, with recovery and return to baseline by Week 6 in most patients1,2
- Lymphopenia occurred in the absence of T-cell depletion1,2
- In clinical trials, 80% of patients treated with TZIELD developed lymphopenia compared to 17% of patients in the control group1
Guidance for lymphopenia1
- Monitor white blood cell counts during the 2-week treatment period
-
If prolonged severe lymphopenia (<500 cells/μL, lasting one week or longer) develops, discontinue TZIELD
Concomitant immunosuppressive medication1
In T1D studies, the safety and efficacy of TZIELD in combination with immunosuppressive medication have note been evaluated. Caution should be exercised when considering concomitant use of immunosuppressive medication.
Serious infections1
Bacterial and viral infections have occurred in TZIELD-treated patients including gastroenteritis, cellulitis, pneumonia, abscess and sepsis. In clinical trials, patients treated with TZIELD had a higher rate of serious infections (3.5%) than patients in the control group (2%).
Management considerations1
Monitor patients for signs and symptoms of infection during and after TZIELD treatment. If serious infection develops, treat appropriately, and discontinue TZIELD.
Hypersensitivity reactions1
Acute hypersensitivity reactions, including serum sickness, angioedema, urticaria, rash, vomiting and bronchospasm occurred in TZIELD-treated patients.
Hypersensitivity reactions were reported with TZIELD in the TN-10 trial. Serum sickness was observed in 2% (1/44) of TZIELD-treated patients compared to 0% (0/32) of placebo-treated patients. See Rash and Hypersensitivity Reactions (section 4.8) of the SmPC for additional safety information from the five pooled clinical trials.
Management considerations1
If severe hypersensitivity reactions occur, discontinue use of TZIELD and treat promptly..
Vaccinations1
The safety of immunisation with live-attenuated vaccine in patients treated with TZIELD has not been studied. TZIELD may interfere with the immune response to vaccination and decrease vaccine efficacy.
Administer all age-appropriate vaccinations prior to starting TZIELD1
- Inactivated or messenger ribonucleic acid (mRNA) vaccinations are not recommended within the 2 weeks prior to TZIELD treatment, during treatment, or 6 weeks after completion of treatment
- Live-attenuated vaccinations are not recommended within the 8 weeks prior to TZIELD treatment, during treatment, or up to 52 weeks after treatment.
List of excipients1
Dibasic sodium phosphate (E339)
Monobasic sodium phosphate (E339)
Polysorbate 80 (E433)
Sodium chloride
Water for injection
Incompatibilities1
In the absence of compatibility studies, TZIELD should not be mixed with other medicinal products. Do not add or simultaneously infuse other medicinal products through the same intravenous line. This medicinal product should be prepared and administered as instructed in section 4.2 and section 6.6 of the SmPC.
Pregnancy1
Available case reports from clinical trials with TZIELD are insufficient to identify a drug-associated risk of major birth defects, miscarriage or other adverse maternal or foetal outcomes.
Although there are no data on TZIELD, monoclonal antibodies can be actively transported across the placenta, and TZIELD may cause immunosuppression in the utero-exposed infant. To minimise exposure to a foetus, avoid use of TZIELD during pregnancy and for at least 30 days prior to planned pregnancy.
Breastfeeding1
There are no data on the presence of TZIELD in human milk, effects on milk production, or effects on the breastfed child.
As endogenous maternal immunoglobulin G (IgG) and monoclonal antibodies are transferred into human milk, a lactating woman may interrupt breastfeeding and pump and discard breast milk during treatment and for 20 days after TZIELD administration to minimise drug exposure to a breastfed child.
Fertility1
There are no clinical data available for TZIELD on the effects on fertility.
Effects on ability to drive and use machines1
Fatigue has been reported in patients taking TZIELD and this should be taken into account when driving or using machines.
Get in Touch with Us
Questions? Leave your details and we'll reach out to you at your preferred time.
Get in touch*Stage 2 is defined as having two or more pancreatic islet autoantibodies (glutamic acid decarboxylase 65 autoantibodies, insulin autoantibody, islet cell autoantibody, insulinoma-associated antigen 2 autoantibody, and zinc transporter 8 autoantibody) and dysglycaemia on OGTT.1
†The recommended dosing as per the SmPC is 65 μg/m2 on Day 1; 125 μg/m2 on Day 2; 250 μg/m2 on Day 3; 500 μg/m2 on Day 4; and 1,030 μg/m2 on Days 5 to 14.1
‡Criteria for Stage 3 T1D were based on glucose testing or the presence of unquivocal hyperglycaemia or hyperglycaemia crisis.4
||Adverse reactions were evaluated in a pool of adult and paediatric patients across one phase 2 study in patients with Stage 2 autoimmune T1D (TN-10), three placebo-controlled studies in an unapproved population, and one open-label standard-of-care controlled study of TZIELD in an unapproved population.1
§The median follow-up time was 80.46 months. P=0.03 Fisher's exact test.3
¶Cannot be estimated from available data.
#Serious infections included cellulitis, gastroenteritis, pneumonia, and wound infection any time during or after the first dose of study treatment.1
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; CRS, cytokine release syndrome; HR, hazard ratio; mRNA, messenger ribonucleic acid; OGTT, oral glucose tolerance test; SmPC, Summary of Product Characteristics; T1D, Type 1 diabetes; ULN, upper limit of normal.
- TZIELD® (teplizumab) UK Summary of Product Characteristics. 2025.
- Herold KC, et al. N Engl J Med. 2019; 381(7): 603–613.
- Lledó-Delgado A, et al. J Clin Invest. 2024; 134(18): e177492.
- American Diabetes Association. Diabetes Care. 2019; 42(Suppl 1): S13–S28.
MAT-XU-2500766 (v1.0) | November 2025
