Consensus guidance for monitoring patients
Regular glycaemic monitoring is recommended by the International Society for Pediatric and Adolescent Diabetes (ISPAD) in all patients who have tested positive for islet AAbs to allow T1D staging, inform education, and provide opportunities for patients to participate in research or receive treatment to delay progression.1
The following Consensus Guidance for monitoring is recommended in patients who test positive for islet AAbs:
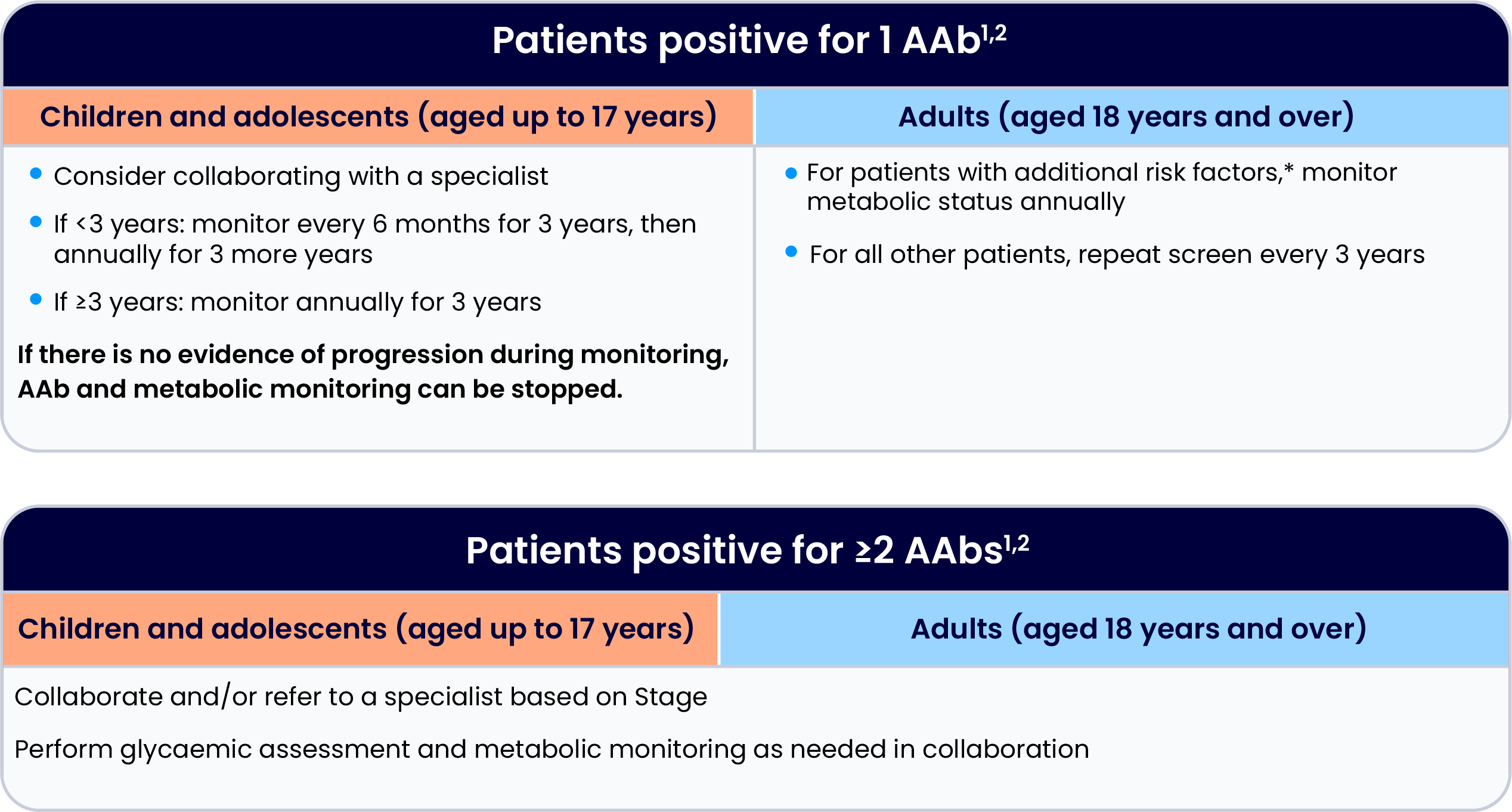
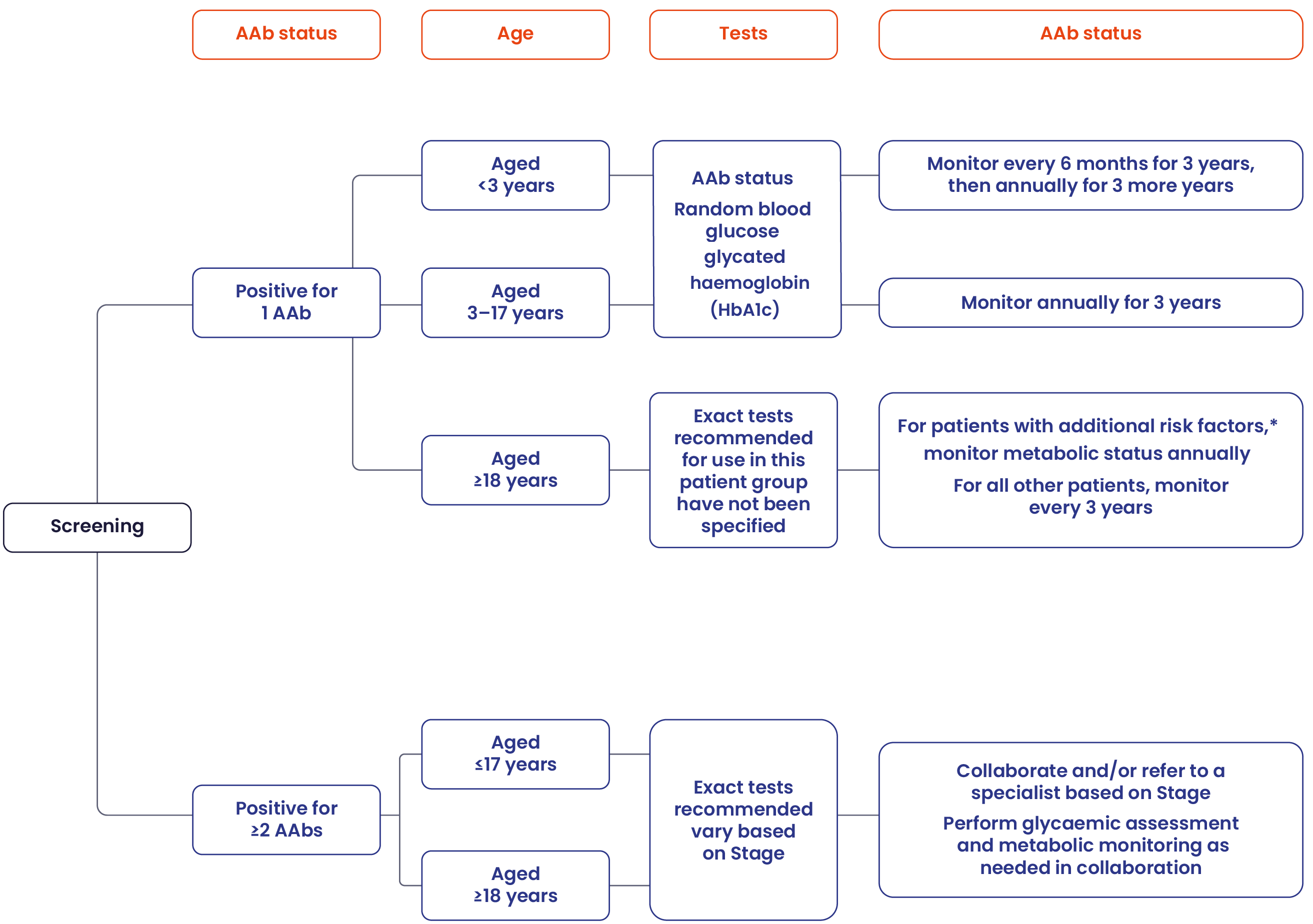
Please refer to the full Consensus Guidance for additional information of monitoring.
Adapted from the 'Consensus Guidance for Monitoring Individuals With Islet-Autoantibody-Positive Pre-Stage 3 Type 1 Diabetes', developed by Breakthrough T1D in conjunction with international experts and societies.2
Testing should be coupled with education and ongoing monitoring for those identified with islet AAbs.1
SNOMED diagnostic codes
Pre-symptomatic T1D (Stage 1 and 2) can be captured on a patient's electronic health record, using a unique SNOMED (Systematized Nomenclature of Medicine) code. Utilisation of this code could unlock better monitoring, follow-up, and education for people in the earliest stages of autoimmune T1D.4
Pre-symptomatic autoimmune T1D (Stages 1 and 2):
1290118005
Stage 3 autoimmune T1D:
46635009
Get in Touch with Us
Questions? Leave your details and we'll reach out to you at your preferred time.
Get in touchINDICATION: TZIELD is indicated to delay the onset of Stage 3 T1D in adult and paediatric patients 8 years of age and older with Stage 2 T1D.3
*Additional risk includes one or more of the following: first-degree relative with T1D, elevated genetic risk for T1D if tested, dysglycaemia (e.g. impaired fasting glucose or impaired glucose tolerance), or history of stress hyperglycaemia.2
AAb, autoantibody; HbA1c, glycated haemoglobin; ISPAD, International Society for Pediatric and Adolescent Diabetes; SNOMED, Systematized Nomenclature of Medicine; T1D, Type 1 diabetes.
- Haller MJ, et al. Horm Res Paediatr. 2024; 97(6): 529–545.
- Phillip M, et al. Diabetes Care. 2024; 47(8): 1276–1298.
- TZIELD® (teplizumab) UK Summary of Product Characteristics. 2025.
- University of Birmingham. Launch of new international medical code for presymptomatic type 1 diabetes. 2024. Available at: https://www.birmingham.ac.uk/news/2024/launch-of-new-international-medical-code-for-presymptomatic-type-1-diabetes. Accessed November 2025.
MAT-XU-2500763 (v1.0) | November 2025