ALPROLIX prophylaxis has extended dosing vs SHLs that helps reduce treatment burden1,2
CONVENIENT STARTING DOSES:
Adult and Adolescent Starting Doses (≥12 years)1

OR

- A 7-day interval is the most common EHL prophylaxis regimen in hemophilia B1,2*
*Data are from an online survey conducted by Sanofi in April 2019 with 359 adult patients and caregivers to provide insights into the US hemophilia A and hemophilia B markets. Ninety respondents were adults with hemophilia B and 60 respondents were caregivers of a patient with hemophilia B.2
Pediatric Starting Dose (≤11 years)1

Flexibility for their growing needs. Simplicity for their caregivers.
- The same once-weekly schedule as other EHLs, so patients and caregivers can switch with confidence3,4
- Dosing schedule may be adjusted based on individual response. More frequent or higher doses may be needed, especially in children under 6 years old1
- On average, 1 IU of ALPROLIX per kg of body weight increases the level of circulating factor IX by approximately 1% in children aged ≥6 years and 0.6% in children aged <6 years1
EXTENDED DOSING OPTIONS: Adults and adolescents (≥12 years)5
14-day interval
- More than half (54%) of patients in the B-LONG clinical trials who started with 10-day dosing (at 100 IU/kg) were able to extend dosing to 14 days or longer6
- The median duration of exposure to ALPROLIX at a ≥14-day dosing interval was 4.3 years, as reported in a post hoc analysis5†
- In the B-YOND trial, most patients were on individualized prophy beforehand and were well controlled before they began extending their time between doses5‡
- 78% (n=18/23) of the patients were able to maintain a dosing schedule of ≥14 days in B-YOND5
- 5 patients returned to <14-day dosing5
- Patients in the Kids B-LONG trial were not included in this later analysis5
†Data are reported from a post hoc analysis of 23 adult and adolescent patients (aged ≥12 years) who received ALPROLIX prophylaxis with a dosing interval of ≥14 days at any time during B-LONG or B-YOND.5
‡From a later analysis of 23 people who extended to 14 days or more during the B-LONG and B-YOND trials.5

With the widest range of options, including a 4000 IU vial, ALPROLIX offers the potential to resolve bleeds with fewer vials1,3,4,7-11

On-demand dosing with ALPROLIX can be used to control your patients' bleeding episodes1
Major and minor on-demand dosage for patients of all ages1 | ||
|
TYPE OF BLEED |
TARGET CIRCULATING FACTOR IX |
DOSING INTERVAL |
|
Minor and moderatea |
30-60 |
Repeat every 48 hours if there is further evidence of bleeding |
|
Majorb |
80-100 |
Consider a repeat dose after 6-10 hours and then every 24 hours for the first 3 days Due to the long half life of ALPROLIX, the dose may be reduced, and the frequency of dosing may be extended after Day 3 to every 48 hours or longer until bleeding stops and healing is achieved |
ALPROLIX reaches peak activity in 10 minutes—as quickly as BeneFIX® [coagulation factor IX (recombinant)]2*†
Studies have not been conducted to assess the safety or efficacy of ALPROLIX compared with BeneFIX.
aMinor and moderate bleed events. For example: uncomplicated hemarthroses, superficial muscle (except iliopsoas) without neurovascular compromise, superficial soft tissue, mucous membranes.1
bMajor bleed events. For example: iliopsoas and deep muscle with neurovascular injury or substantial blood loss; pharyngeal; retropharyngeal; retroperitoneal; CNS.1
*A subset of 22 patients (the sequential pharmacokinetic subgroup) received consecutive single IV doses of 50 IU/kg BeneFIX and ALPROLIX at the beginning of the B-LONG study (baseline) for direct comparison. For both ALPROLIX and BeneFIX, peak activity was reached approximately 10 minutes after the start of the infusion.2,6
†Peak activity level does not mean bleeds are resolved within 10 minutes.2
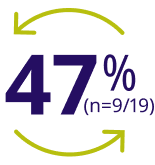
47% of patients ≥12 years treated with ALPROLIX on-demand during the B-LONG trial switched to ALPROLIX prophylaxis during the B-YOND trial16
Perioperative dosing offers hemostatic control for minor and major surgeries1
Major and minor perioperative dosage for patients of all ages1 | ||
|
TYPE OF SURGERY |
TARGET CIRCULATING FACTOR IX |
DOSING INTERVAL |
|
Minor |
50-80 |
A single infusion may be sufficient. Repeat as needed after 24-48 hours until healing is achieved |
|
Major |
60-100 |
Consider a repeat dose after 6-10 hours and then every 24 hours for the first 3 days Due to the long half life of ALPROLIX, the dose may be reduced and post-operative dosing may be extended after Day 3 to every 48 hours or longer until healing is achieved |
- Thirty-five major surgeries were performed in 22 patients during B-LONG and B-YOND1
- An additional 62 minor surgeries were performed in 37 patients during B-LONG, Kids B-LONG, and B-YOND1
ALPROLIX prophylaxis has extended dosing vs SHLs that helps reduce treatment burden1,2
CONVENIENT STARTING DOSES:
Adult and Adolescent Starting Doses (≥12 years)1

OR

- A 7-day interval is the most common EHL prophylaxis regimen in hemophilia B1,2*
*Data are from an online survey conducted by Sanofi in April 2019 with 359 adult patients and caregivers to provide insights into the US hemophilia A and hemophilia B markets. Ninety respondents were adults with hemophilia B and 60 respondents were caregivers of a patient with hemophilia B.2
Pediatric Starting Dose (≤11 years)1

Flexibility for their growing needs. Simplicity for their caregivers.
- The same once-weekly schedule as other EHLs, so patients and caregivers can switch with confidence3,4
- Dosing schedule may be adjusted based on individual response. More frequent or higher doses may be needed, especially in children under 6 years old1
- On average, 1 IU of ALPROLIX per kg of body weight increases the level of circulating factor IX by approximately 1% in children aged ≥6 years and 0.6% in children aged <6 years1
EXTENDED DOSING OPTIONS: Adults and adolescents (≥12 years)5
14-day interval

- More than half (54%) of patients in the B-LONG clinical trials who started with 10-day dosing (at 100 IU/kg) were able to extend dosing to 14 days or longer6
- The median duration of exposure to ALPROLIX at a ≥14-day dosing interval was 4.3 years, as reported in a post hoc analysis5†
- In the B-YOND trial, most patients were on individualized prophy beforehand and were well controlled before they began extending their time between doses5‡
- 78% (n=18/23) of the patients were able to maintain a dosing schedule of ≥14 days in B-YOND5
- 5 patients returned to <14-day dosing5
- Patients in the Kids B-LONG trial were not included in this later analysis5
†Data are reported from a post hoc analysis of 23 adult and adolescent patients (aged ≥12 years) who received ALPROLIX prophylaxis with a dosing interval of ≥14 days at any time during B-LONG or B-YOND.5
‡From a later analysis of 23 people who extended to 14 days or more during the B-LONG and B-YOND trials.5

With the widest range of options, including a 4000 IU vial, ALPROLIX offers the potential to resolve bleeds with fewer vials1,3,4,7-11

On-demand dosing with ALPROLIX can be used to control your patients' bleeding episodes1
Major and minor on-demand dosage for patients of all ages1 | ||
|
TYPE OF BLEED |
TARGET CIRCULATING FACTOR IX |
DOSING INTERVAL |
|
Minor and moderatea |
30-60 |
Repeat every 48 hours if there is further evidence of bleeding |
|
Majorb |
80-100 |
Consider a repeat dose after 6-10 hours and then every 24 hours for the first 3 days Due to the long half life of ALPROLIX, the dose may be reduced, and the frequency of dosing may be extended after Day 3 to every 48 hours or longer until bleeding stops and healing is achieved |
ALPROLIX reaches peak activity in 10 minutes—as quickly as BeneFIX® [coagulation factor IX (recombinant)]2*†
Studies have not been conducted to assess the safety or efficacy of ALPROLIX compared with BeneFIX.
aMinor and moderate bleed events. For example: uncomplicated hemarthroses, superficial muscle (except iliopsoas) without neurovascular compromise, superficial soft tissue, mucous membranes.1
bMajor bleed events. For example: iliopsoas and deep muscle with neurovascular injury or substantial blood loss; pharyngeal; retropharyngeal; retroperitoneal; CNS.1
*A subset of 22 patients (the sequential pharmacokinetic subgroup) received consecutive single IV doses of 50 IU/kg BeneFIX and ALPROLIX at the beginning of the B-LONG study (baseline) for direct comparison. For both ALPROLIX and BeneFIX, peak activity was reached approximately 10 minutes after the start of the infusion.2,6
†Peak activity level does not mean bleeds are resolved within 10 minutes.2
47% of patients ≥12 years treated with ALPROLIX on-demand during the B-LONG trial switched to ALPROLIX prophylaxis during the B-YOND trial16

Perioperative dosing offers hemostatic control for minor and major surgeries1
Major and minor perioperative dosage for patients of all ages1 | ||
|
TYPE OF SURGERY |
TARGET CIRCULATING FACTOR IX |
DOSING INTERVAL |
|
Minor |
50-80 |
A single infusion may be sufficient. Repeat as needed after 24-48 hours until healing is achieved |
|
Major |
60-100 |
Consider a repeat dose after 6-10 hours and then every 24 hours for the first 3 days Due to the long half life of ALPROLIX, the dose may be reduced and post-operative dosing may be extended after Day 3 to every 48 hours or longer until healing is achieved |
- Thirty-five major surgeries were performed in 22 patients during B-LONG and B-YOND1
- An additional 62 minor surgeries were performed in 37 patients during B-LONG, Kids B-LONG, and B-YOND1
Clinical Trial Information
B-LONG was a phase 3 open-label study investigating the safety and efficacy of ALPROLIX in 123 adult and adolescent PTPs aged ≥12 years with severe hemophilia B. Study arms included: fixed-interval (weekly) (n=63), fixed-dose (interval-adjusted) (n=29), episodic (on-demand) (n=27), and surgical (n=12).1
Kids B-LONG was a phase 3 open-label study investigating the safety and efficacy of ALPROLIX in 30 PTPs aged ≤11 years with severe hemophilia. The number of patients 1 to 5 years of age was 15, and 6 to 11 years of age was 15. All 30 patients were treated with ALPROLIX on an individualized prophylactic regimen.1
B-YOND was an open-label extension trial that studied the long-term safety and efficacy of ALPROLIX over 5 years in 120 adult, adolescent, and pediatric patients previously treated in Kids B-LONG or B-LONG. Study arms included: fixed-interval (n=74), fixed-dose (n=36), modified prophylaxis (n=17), and episodic (on-demand) (n=15).12
Why flexible dosing matters in hemophilia B treatment
It’s important for patients to have a treatment that can fit their needs over time, because treatment journeys take many forms

of adult patients with severe hemophilia B switch their treatment approach to accommodate changes in their lifestyle13
Changes in activity
Patients who treat on-demand may recognize the need to transition to prophylaxis14
Life change
Ongoing prophylaxis may feel burdensome during early adulthood or other transitional life stages, leading to changes in treatment approach15
While the World Federation of Hemophilia recommends use of prophylaxis over on-demand therapy, ALPROLIX can meet your patients where they are, with experience across ages and settings1,14
Protection,§ Experience, and Dosing: a discussion with Dr De Angulo
Dr Guillermo De Angulo shares his clinical experience with ALPROLIX.
Hear about how he integrates ALPROLIX into his practice and how he evaluates his patients’ ongoing treatment.
§ALPROLIX has been proven to help patients prevent bleeding episodes using a prophylaxis regimen.1
CNS=central nervous system; EHL=extended half-life; PTP=previously treated patient; SHL=standard half-life.
INDICATION:
References: 1. ALPROLIX. Package insert. Bioverativ Therapeutics Inc; 2023. 2. Data on file. Waltham, MA; Bioverativ Therapeutics Inc. 3. Rebinyn. Package insert. Novo Nordisk Inc; 2022. 4. Idelvion. Package insert. CSL Behring GmbH; 2023. 5. Shapiro AD, Kulkarni RD, Ragni MV, et al. Post hoc longitudinal assessment of efficacy and safety of recombinant factor IX Fc fusion protein in hemophilia B. Blood Adv. 2023;7(13):3049-3057. 6. Powell JS, Pasi KJ, Ragni MV, et al; B-LONG Investigators. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N Engl J Med. 2013;369(24):2313-2323. 7. Alphanine SD. Package insert. Grifols Biologicals Inc; 2022. 8. Benefix. Package insert. Wyeth Pharmaceuticals Inc; 2022. 9. Ixinity. Package insert. Medexus Pharma, Inc; 2024. 10. Profilnine SD. Package insert. Grifols Biologicals Inc; 2021. 11. Rixubis. Package insert. Takeda Pharmaceuticals U.S.A. Inc; 2025. 12. Pasi KJ, Fischer K, Ragni M, et al. Long-term safety and sustained efficacy for up to 5 years of treatment with recombinant factor IX Fc fusion protein in subjects with haemophilia B: results from the B-YOND extension study. Haemophilia. 2020;26(6):e262-e271. 13. Baumann K, Hernandez G, Witkop M, et al. Impact of mild to severe hemophilia on engagement in recreational activities by US men, women, and children with hemophilia B: the bridging hemophilia B experiences, results and opportunities into solutions (B-HERO-S) study. Eur J Haematol. 2017;98(suppl 86):25-34. 14. Srivastava A, Santagostino E, Dougall A, et al; WFH Guidelines for the Management of Hemophilia panelists and co-authors. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(suppl 6):1-158. 15. Thornburg CD, Duncan NA. Treatment adherence in hemophilia. Patient Prefer Adherence. 2017;11:1677-1686. 16. Pasi KJ, Fischer K, Ragni M, et al. Long-term safety and efficacy of extended-interval prophylaxis with recombinant factor IX Fc fusion protein (rFIXFc) in subjects with haemophilia B. Thromb Haemost. 2017;117(3):508-518.

