We’re committed to helping support ALPROLIX patients every step of the way
Billing and coding information
The codes listed below are provided for informational purposes only and do not constitute legal or reimbursement advice. They are not intended to substitute for or influence the independent medical judgment of the prescriber. The codes may not apply to all patients or to all health plans, and additional codes not listed may apply to some patients. The treating physician is solely responsible for diagnosis, coding and determination of the appropriate ICD-10-CM codes that describe the patient’s condition and are supported by the medical record.
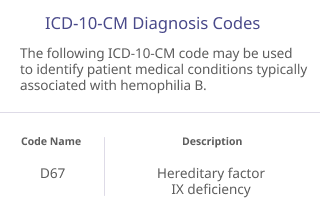
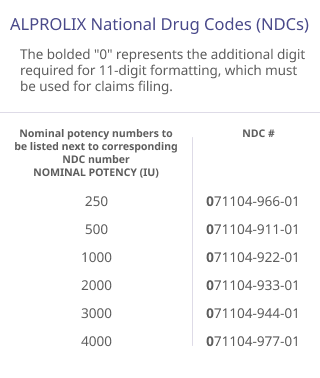
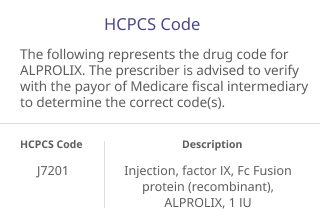
The ICD-10-CM and HCPCS codes provided are based on AMA or CMS guidelines. Use of the information above does not guarantee that the payor will provide coverage for ALPROLIX. Because government and other third-party payor coding requirements change periodically, please verify current coding requirements directly with the payor being billed.
Support for your patients from Sanofi Support | HemAssist
We complement the support you provide your ALPROLIX patients and their caregivers with access to treatment, education, and financial assistance from HemAssist Sanofi Support.
Our support
.png)
Financial assistance
Every person's situation is different, which is why we work directly with patients to help identify the financial assistance programs for which they may be eligible.
.png)
Treatment education
We can help your patients understand more about ALPROLIX, as well as offer supplemental administration training and tips to help them start and stay on track with treatment as prescribed.
.png)
Access to treatment
From navigating insurance coverage to assisting with reimbursement, our team works with you and your patients to get them started on ALPROLIX as efficiently as possible.
Team members
Caring for ALPROLIX patients may require some support. That's why you have a dedicated HemAssist team here to assist you. Call 1-833-723-5463 Monday – Friday 8 AM to 7 PM ET.
Clinical Educator (CE)
Clinical Educators can answer questions about your patient’s condition and treatment and can provide helpful support, including disease state education, treatment tips, and administration training.
Case Manager
Your Case Manager will provide you with personalized insurance investigations and answer your specific questions about financial assistance options for eligible patients.
Field Reimbursement Manager (FRM)
Your FRM can help you navigate insurance coverage and provide reimbursement education and solutions.
HemAssist Patient Enrollment Form
Complete the form with your patient to enroll them in HemAssist, the patient support program for ALPROLIX.

Need help enrolling your patients?
It takes just a few steps. Learn how to get started. Call our team at 1-833-723-5463 Monday - Friday 8 AM to 7 PM ET with questions.
ALPROLIX instructions for use
This document contains information on how to use ALPROLIX, including reconstitution, pooling, administration, and storage.
10 years trusted
This video, created for the 10th anniversary of the FDA approval of ALPROLIX, features physicians, patients, and community leaders sharing the impact ALPROLIX has had over the years.
Explore our educational resources to learn more about ALPROLIX from those who prescribe it
Peer Perspectives: 10 Years of ALPROLIX
Switching EHL patients to ALPROLIX: A prophylaxis case study
Dr Guy Young shares his experience with pediatric and adolescent patients, and what guides a switch to ALPROLIX from another EHL treatment.
ALPROLIX Clinical Experience:
A conversation with Dr Amy Shapiro, MD, and Dr Lynn Malec, MD
Protection,* Experience, and Dosing: A Discussion With Dr De Angulo
Hear about how Dr De Angulo integrates ALPROLIX into his practice and how he evaluates his patients’ ongoing treatment.
*ALPROLIX has been proven to help patients prevent bleeding episodes using a prophylaxis regimen.1

The Factor Forum is an engaging podcast series for healthcare professionals that provides peer insights and experiences that have helped them enhance treatment for their patients with hemophilia B. The series dives deep into topics, including treatment approaches, comorbidity management, patient cases, and the benefits of a multidisciplinary care team.
FACTORING IN SHLs AND EHLs FOR HEMOPHILIA B PATIENTS
Looks at the evolution of factor treatment and how EHLs have changed the hemophilia treatment landscape by optimizing care
AN APPROACH TO COMPLEX CASES IN HEMOPHILIA B
Explores common comorbidities and how choosing the right treatment regimen can impact patient outcomes and adherence
We’re committed to helping support ALPROLIX patients every step of the way
Billing and coding information
The codes listed below are provided for informational purposes only and do not constitute legal or reimbursement advice. They are not intended to substitute for or influence the independent medical judgment of the prescriber. The codes may not apply to all patients or to all health plans, and additional codes not listed may apply to some patients. The treating physician is solely responsible for diagnosis, coding and determination of the appropriate ICD-10-CM codes that describe the patient’s condition and are supported by the medical record.



The ICD-10-CM and HCPCS codes provided are based on AMA or CMS guidelines. Use of the information above does not guarantee that the payor will provide coverage for ALPROLIX. Because government and other third-party payor coding requirements change periodically, please verify current coding requirements directly with the payor being billed.
Support for your patients from Sanofi Support | HemAssist
We complement the support you provide your ALPROLIX patients and their caregivers with access to treatment, education, and financial assistance from HemAssist Sanofi Support.
Our support
.png)
Financial assistance
Every person's situation is different, which is why we work directly with patients to help identify the financial assistance programs for which they may be eligible.
.png)
Treatment education
We can help your patients understand more about ALPROLIX, as well as offer supplemental administration training and tips to help them start and stay on track with treatment as prescribed.
.png)
Access to treatment
From navigating insurance coverage to assisting with reimbursement, our team works with you and your patients to get them started on ALPROLIX as efficiently as possible.
Team members
Caring for ALPROLIX patients may require some support. That's why you have a dedicated HemAssist team here to assist you. Call 1-833-723-5463 Monday – Friday 8 AM to 7 PM ET.
Clinical Educator (CE)
Clinical Educators can answer questions about your patient’s condition and treatment and can provide helpful support, including disease state education, treatment tips, and administration training.
Case Manager
Your Case Manager will provide you with personalized insurance investigations and answer your specific questions about financial assistance options for eligible patients.
Field Reimbursement Manager (FRM)
Your FRM can help you navigate insurance coverage and provide reimbursement education and solutions.
HemAssist Patient Enrollment Form
Complete the form with your patient to enroll them in HemAssist, the patient support program for ALPROLIX.

Need help enrolling your patients?
It takes just a few steps. Learn how to get started. Call our team at 1-833-723-5463 Monday - Friday 8 AM to 7 PM ET with questions.
ALPROLIX instructions for use
This document contains information on how to use ALPROLIX, including reconstitution, pooling, administration, and storage.
10 years trusted
This video, created for the 10th anniversary of the FDA approval of ALPROLIX, features physicians, patients, and community leaders sharing the impact ALPROLIX has had over the years.
Explore our educational resources to learn more about ALPROLIX from those who prescribe it
Peer Perspectives: 10 Years of ALPROLIX
Switching EHL patients to ALPROLIX: A prophylaxis case study
Dr Guy Young shares his experience with pediatric and adolescent patients, and what guides a switch to ALPROLIX from another EHL treatment.
ALPROLIX Clinical Experience:
A conversation with Dr Amy Shapiro, MD, and Dr Lynn Malec, MD
Protection,* Experience, and Dosing: A Discussion With Dr De Angulo
Hear about how Dr De Angulo integrates ALPROLIX into his practice and how he evaluates his patients’ ongoing treatment.
*ALPROLIX has been proven to help patients prevent bleeding episodes using a prophylaxis regimen.1

The Factor Forum is an engaging podcast series for healthcare professionals that provides peer insights and experiences that have helped them enhance treatment for their patients with hemophilia B. The series dives deep into topics, including treatment approaches, comorbidity management, patient cases, and the benefits of a multidisciplinary care team.
FACTORING IN SHLs AND EHLs FOR HEMOPHILIA B PATIENTS
Looks at the evolution of factor treatment and how EHLs have changed the hemophilia treatment landscape by optimizing care
AN APPROACH TO COMPLEX CASES IN HEMOPHILIA B
Explores common comorbidities and how choosing the right treatment regimen can impact patient outcomes and adherence
AMA=American Medical Association; CMS=Centers for Medicare & Medicaid Services; EHL=extended half-life; HCPCS=healthcare common procedure coding system; ICD-10-CM=international classification of diseases, 10th revision, clinical modification; SHL=standard half-life.
INDICATION:
Reference: 1. ALPROLIX [package insert]. Waltham, MA: Bioverativ Therapeutics Inc.
References for Factoring in SHLs and EHLs for Hemophilia B Patients for Factor Forum Podcast:
-
ALPROLIX [package insert]. Waltham, MA: Bioverativ Therapeutics Inc.
-
Björkman S. Population pharmacokinetics of recombinant factor IX: implications for dose tailoring. Haemophilia. 2013;19(5):753-757.
-
Data on file. Waltham, MA; Bioverativ Therapeutics Inc.
-
Diao L, Li S, Ludden T, Gobburu J, Nestorov I, Jiang H. Population pharmacokinetic modelling of recombinant factor IX Fc fusion protein (rFIXFc) in patients with haemophilia B. Clin Pharmacokinet. 2014;53(5):467-477.
-
Geraghty S, Dunkley T, Harrington C, Lindvall K, Maahs J, Sek J. Practice patterns in haemophilia A therapy–global progress towards optimal care. Haemophilia. 2006;12(1):75-81.
-
Gui T, Lin HF, Jin DY, et al. Circulating and binding characteristics of wild-type factor IX and certain Gla domain mutants in vivo. Blood. 2002;100(1):153-158.
-
Hacker M, Messer WS, Bachmann KA. Pharmacology: Principles and Practice. 1st ed. Academic Press; 2009.
-
Iorio A, Fischer K, Blanchette V, Rangarajan S, Young G, Morfini M; Pharmacokinetic (PK) Expert Working Group of the International Prophylaxis Study Group (the IPSG). Tailoring treatment of haemophilia B: accounting for the distribution and clearance of standard and extended half-life FIX concentrates. Thromb Haemost. 2017;117(6):1023-1030.
-
Kachalsky E, Geary M, Pennick L, et al. The role of the hemophilia treatment center social worker in the United States. Poster presented at: WFH 2016 World Congress; July 24-28, 2016; Orlando, Florida.
-
Lenting PJ, Schooten CJM van, Denis CV. Clearance mechanisms of von Willebrand factor and factor VIII. J Thromb Haemost. 2007;5(7):1353-1360.
-
Lock J, de Bekker-Grob EW, Urhan G, et al; 'OPTI-CLOT' study group. Facilitating the implementation of pharmacokinetic-guided dosing of prophylaxis in haemophilia care by discrete choice experiment. Haemophilia. 2016;22(1):e1-e10.
-
Morfini M. The history of clotting factor concentrates pharmacokinetics. J Clin Med. 2017;6(3):35.
-
Pasi KJ, Fischer K, Ragni M, et al. Long-term safety and efficacy of extended-interval prophylaxis with recombinant factor IX Fc fusion protein (rFIXFc) in subjects with haemophilia B. Thromb Haemost. 2017;117(3):508-518.
-
Pasi KJ, Fischer K, Ragni M, et al. Long-term safety and sustained efficacy for up to 5 years of treatment with recombinant factor IX Fc fusion protein in subjects with haemophilia B: results from the B-YOND extension study. Haemophilia. 2020;26(6):e262-e271.
-
Powell JS, Pasi KJ, Ragni MV, et al; B-LONG Investigators. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N Engl J Med. 2013;369(24):2313-2323.
-
Srivastava A, Santagostino E, Dougall A, et al; WFH Guidelines for the Management of Hemophilia panelists and co-authors. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(suppl 6):1-158.
-
Swiech K, Picanço-Castro V, Covas DT. Production of recombinant coagulation factors: are humans the best host cells? Bioengineered. 2017;8(5):462-470.
-
Torres-Ortuño A, Cuesta-Barriuso R, Nieto-Munuera J. Parents of children with haemophilia at an early age: assessment of perceived stress and family functioning. Haemophilia. 2014;20(6):756-762.
-
van Os S, Ryder N, Hart DP, Troop N. Adherence to prophylaxis in adolescents and young adults with severe haemophilia: a qualitative study with healthcare professionals. Health Psychol Behav Med. 2020;8(1):55-72.
References for An Approach to Complex Cases in Hemophilia B for Factor Forum Podcast:
-
ALPROLIX [package insert]. Waltham, MA: Bioverativ Therapeutics Inc.
-
Astermark J, Hermans C, Ezzalfani M, et al. rFIXFc prophylaxis improves pain and levels of physical activity in haemophilia B: post hoc analysis of B-LONG using haemophilia-specific quality of life questionnaires. Haemophilia. 2022;28(1):18-26.
-
Data on file. Waltham, MA; Bioverativ Therapeutics Inc.
-
Kachalsky E, Geary M, Pennick L, et al. The role of the hemophilia treatment center social worker in the United States. Poster presented at: WFH 2016 World Congress; July 24-28, 2016; Orlando, Florida.
-
Lewandowska M, Nasr S, Shapiro AD. Therapeutic and technological advancements in haemophilia care: quantum leaps forward. Haemophilia. 2022;28(suppl 4):77-92.
-
Nolan B, Klukowska A, Shapiro A, et al. Final results of the PUPs B-LONG study: evaluating safety and efficacy of rFIXFc in previously untreated patients with hemophilia B. Blood Adv. 2021;5(13):2732-2739.
-
Pasi KJ, Fischer K, Ragni M, et al. Long-term safety and sustained efficacy for up to 5 years of treatment with recombinant factor IX Fc fusion protein in subjects with haemophilia B: results from the B-YOND extension study. Haemophilia. 2020;26(6):e262-e271.
-
Powell JS, Pasi KJ, Ragni MV, et al; B-LONG Investigators. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N Engl J Med. 2013;369(24):2313-2323.
-
Shapiro AD, Kulkarni RD, Ragni MV, et al. Post hoc longitudinal assessment of efficacy and safety of recombinant factor IX Fc fusion protein in hemophilia B. Blood Adv. Published online February 27, 2023. doi:10.1182/bloodadvances.2022009230
-
Srivastava A, Santagostino E, Dougall A, et al; WFH Guidelines for the Management of Hemophilia panelists and co-authors. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(suppl 6):1-158.
-
Stoffman J, Andersson NG, Branchford B, et al. Common themes and challenges in hemophilia care: a multinational perspective. Hematology. 2019;24(1):39-48.
