.png)
SEE aTTP/iTTP |
START CABLIVI,* |
STOP MICROTHROMBI FORMATION3 |
| Diagnosis through clinical assessment | Consider early administration of CABLIVI in combination with PEX and immunosuppressive therapy | While awaiting ADAMTS13 results |
*A conditional recommendation defined as desirable effects of the recommendation outweighing the undesirable effects. Assumes timely access to ADAMTS13 testing and clinical diagnosis based on high likelihood of aTTP/iTTP. If ADAMTS13 testing is not available, do not add CABLIVI.
2025 ISTH Guidelines updated: more evidence supports CABLIVI.3
CABLIVI treatment should start as early as possible (ideally within 3 days), as it blocks microthrombi formation in the absence of ADAMTS133
ISTH Guidelines acknowledge the growing body of evidence for CABLIVI3*:
CABLIVI† may accelerate recovery, shorten hospital stays, and reduce the number of PEX sessions and exacerbations
Adding CABLIVI to PEX and immunosuppressive therapy may be more cost-effective for managing aTTP/iTTP than PEX alone
ISTH Guidelines are intended to describe common clinical considerations and/or procedural steps for the use of referenced products but may not be appropriate for every patient or case. Physicians will assess the patient’s individualized needs for medical care. Assessments appropriate to individual patients may vary depending upon the clinical judgment of the treating physician, and the patient’s need. Sanofi does not promote or encourage the use of its products outside their FDA-approved labeling.
†In combination with PEX and immunosuppressive therapy.
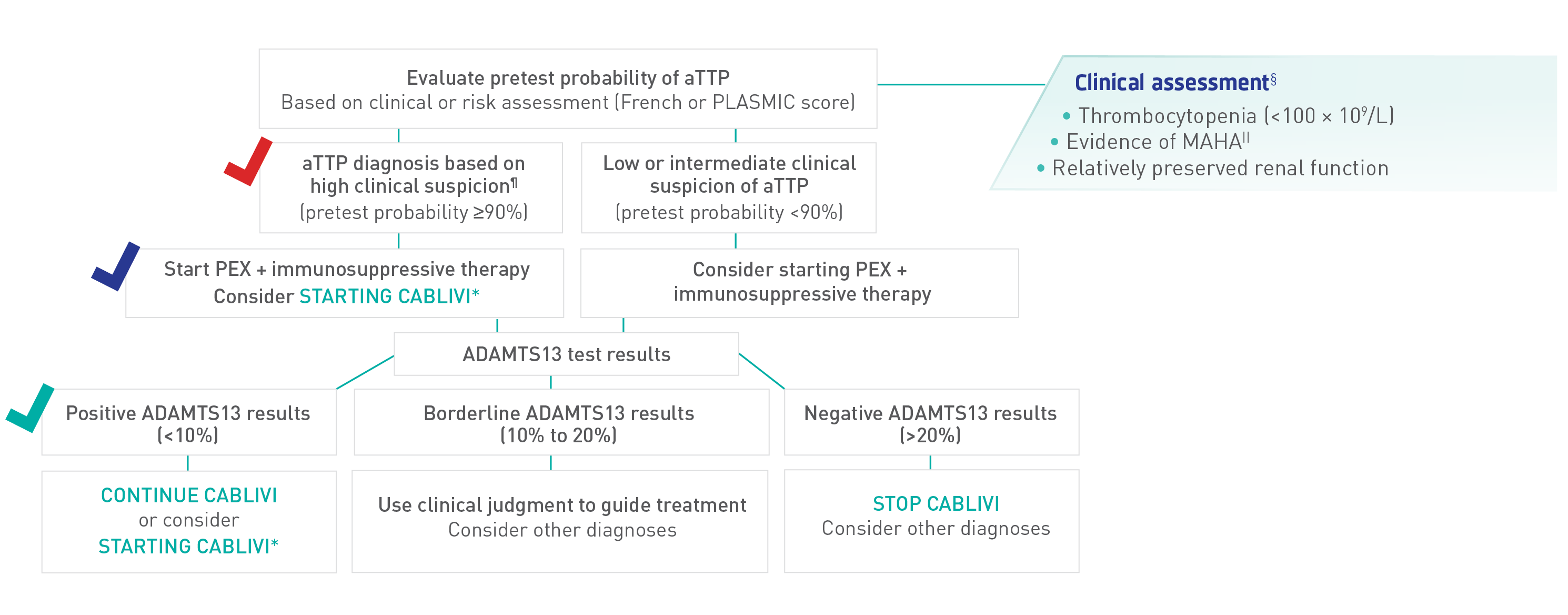
Identifying aTTP/iTTP is crucial for initiation of an appropriate therapeutic strategy2
Clinical assessment2 | OR |
Risk assessment tools‡ |
|
Patient presentation prompting suspicion of aTTP:
|
Available risk assessment tools include:
The higher the risk assessment score, the more likely patients have severe ADAMTS13 deficiency and aTTP/iTTP |
‡ISTH did not appraise the evidence for these 2 tools.
Recommended diagnostic and management strategy for initial, acute events with access to ADAMTS13 results within 7 days
Who should not start CABLIVI?
- CABLIVI is contraindicated in patients with a previous severe hypersensitivity reaction to caplacizumab-yhdp or to any of its excipients
- Withhold CABLIVI treatment 7 days prior to elective surgery, dental procedures, or other invasive interventions
*A conditional recommendation defined as desirable effects of the recommendation outweighing the undesirable effects. Assumes timely access to ADAMTS13 testing and clinical diagnosis based on high likelihood of aTTP/iTTP. If ADAMTS13 testing is not available, do not add CABLIVI.
§List includes laboratory tests and results only; exclusive of physical symptoms, such as petechiae.
||Hb and hematocrit below reference range, low haptoglobin, elevated LDH, presence of schistocytes in peripheral blood smear.
¶ISTH guidelines assume high likelihood of aTTP/iTTP based on clinical assessment or a formal clinical risk assessment tool (French or PLASMIC score) and timely access to ADAMTS13 (within 72 hours).
Adapted from ISTH Guidelines for Diagnosis of TTP.
According to the ISTH TTP Guidelines, treatment of relapses for a patient previously diagnosed with aTTP/iTTP could be started safely based on clinical grounds without the need for a confirmatory ADAMTS13 test2
Timely access to ADAMTS13 results is key to providing optimal care for patients with aTTP/iTTP
Select US labs testing ADAMTS13 activity, inhibitors, and antibodies:
These listings do not constitute an endorsement by Sanofi and are not included in the ISTH Guidelines. The above is a selection of national laboratories offering ADAMTS13 tests for activity, inhibitor, and antibody testing. This is not an exhaustive list of labs that offer one or more of these tests or an endorsement of any lab. Other testing options may be available, including at local or regional laboratories. Content is current as of July 2020, and tests may not be available in all states. Please call laboratory to confirm test availability, sample shipping information, and all other logistics.
Who should not start CABLIVI?
- CABLIVI is contraindicated in patients with a previous severe hypersensitivity reaction to caplacizumab-yhdp or to any of its excipients
- Withhold CABLIVI treatment 7 days prior to elective surgery, dental procedures, or other invasive interventions
ADAMTS13=a disintegrin and metalloproteinase with a thrombospondin type 1 motif, 13; aTTP/iTTP=acquired/immune-mediated thrombotic thrombocytopenic purpura; Hb=hemoglobin; ISTH=International Society on Thrombosis and Haemostasis; LDH=lactate dehydrogenase; MAHA=microangiopathic hemolytic anemia; PEX=plasma exchange.
INDICATION
References: 1. Zheng XL, Vesely SK, Cataland SR, et al. ISTH guidelines for treatment of thrombotic thrombocytopenic purpura. J Thromb Haemost. 2020;18(10):2496-2502. doi:10.1111/jth.15010 2. Zheng XL, Vesely SK, Cataland SR, et al. ISTH guidelines for the diagnosis of thrombotic thrombocytopenic purpura. J Thromb Haemost. 2020;18(10):2486-2495. doi:10.1111/jth.15006 3. Zheng XL, Al‑Housni Z, Cataland SR, et al; International Society on Thrombosis and Haemostasis. 2025 focused update of the 2020 ISTH guidelines for management of thrombotic thrombocytopenic purpura. J Thromb Haemost. 2025;S1538-7836(25)00360-5. doi:10.1016/j.jtha.2025.06.002
.png)