The efficacy and safety of CABLIVI were established in one of the largest acquired/immune-mediated thrombotic thrombocytopenic purpura (aTTP/iTTP) clinical studies1,2
HERCULES was a pivotal, phase 3, double-blind, randomized controlled trial of 145 patients with aTTP/iTTP assessing the efficacy and safety of CABLIVI in combination with PEX and immunosuppressive therapy.
*In combination with PEX and immunosuppressive therapy.
†ISTH guidelines conditionally recommend CABLIVI in combination with PEX and immunosuppressive therapy. Conditional recommendation defined as desirable effects of following the recommendation probably outweighing the undesirable effects.3
‡ISTH guidelines assume high likelihood of aTTP/iTTP based on clinical assessment or a formal clinical risk assessment tool (French or PLASMIC score) and timely access to ADAMTS13 (within 72 hours).3
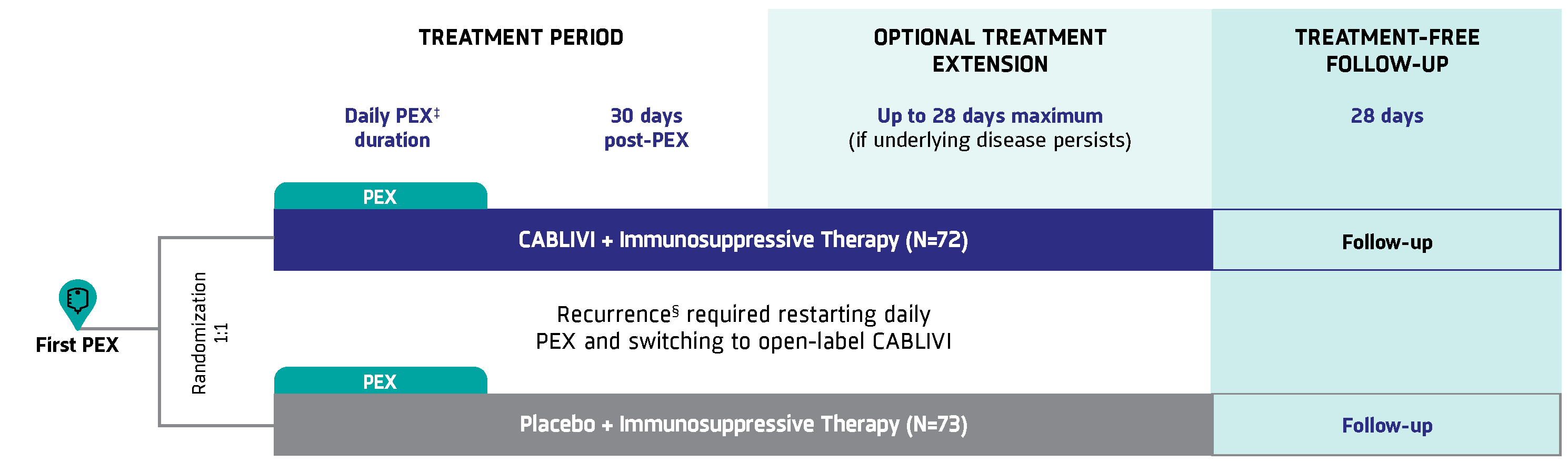
Study design
A pivotal, phase 3, double-blind trial of 145 adult patients randomized to receive CABLIVI, PEX, and immunosuppressive therapy (72) compared with placebo, PEX, and immunosuppressive therapy (73) for the duration of daily PEX and 30 days thereafter. Patients could receive extended treatment for up to 28 days if signs of underlying disease persisted, such as suppressed ADAMTS13 activity levels. There were 2 patients in each group that did not receive any immunosuppressive therapy.1,2
.png)
Key efficacy endpoints1,2
Primary endpoint: Time to platelet count normalization‖
Secondary endpoint: Composite of aTTP/iTTP-related events during study-drug period, including: aTTP/iTTP-related death, recurrence,§ and ≥1 major TE event
Secondary endpoint: Recurrence during overall study period§
‡Daily PEX was variable based on platelet count normalization at ≥150,000/μL and physician discretion.2
§Thrombocytopenia after initial recovery of platelet count (platelet count ≥150,000/μL) that required reinitiation of daily PEX was considered a recurrence. Recurrences were termed exacerbations if they occurred within 30 days of the last PEX and relapses if they occurred more than 30 days after the last PEX.2
‖Platelet count normalization was defined as platelet count ≥150,000/μL with discontinuation of daily PEX 5 days thereafter.2
Select demographic and baseline characteristics in the study population2
| CABLIVI | Placebo | Total | |
| Characteristic | n (%) | n (%) | n (%) |
IMMUNOSUPPRESSIVE THERAPY—NO. (%) | |||
| Glucocorticoids | 69 (96) | 71 (97) | 140 (97) |
| Rituximab | 28 (39) | 35 (48) | 63 (43) |
| Other¶ | 12 (16) | 3 (3) | 15 (10) |
PRESENTING EPISODE OF TTP—NO. (%)# | |||
| Initial | 48 (67) | 34 (47) | 82 (57) |
| Recurrent | 24 (33) | 39 (53) | 63 (43) |
ADAMTS13 ACTIVITY—NO. (%)** | |||
| <10% | 58 (81) | 65 (89) | 123 (85) |
| ≥10% | 13 (18) | 7 (10) | 20 (14) |
Key inclusion criteria: ≥18 years of age; clinical diagnosis of aTTP/iTTP; required initiation of daily PEX and received PEX prior to randomization.
Key exclusion criteria: Platelet count ≥100 × 109/L or >30 × 109/L if serum creatinine level >200 µmol/L; known other causes of thrombocytopenia, such as congenital TTP, pregnancy, or breastfeeding; clinically significant active bleeding or high risk of bleeding; known chronic treatment with anticoagulants; malignant arterial hypertension; life expectancy <6 months.
¶Other immunosuppressive therapies include, for CABLIVI and placebo, respectively: mycophenolate mofetil (6, 0), hydroxychloroquine (2, 1), bortezomib (2, 0), cyclophosphamide (1, 1), cyclosporin (1, 1).
#The difference between the trial groups in the percentage of patients who presented with an initial episode as compared with a recurrent episode was significant (P<0.05).2
**The normal range of ADAMTS13 activity used in the trial was 50% to 130%. As a result of the requirement for previous plasma exchange, baseline ADAMTS13 activity was higher than that measured locally at the time of admission in some cases. When available, ADAMTS13 activity levels that were locally measured at the time of admission were obtained, and the lower value of the baseline and admission values is represented.2
Efficacy
In combination with PEX and immunosuppressive therapy,
CABLIVI helped to normalize platelet counts faster, reduced potentially serious aTTP/iTTP-related events, and had significantly fewer recurrences requiring reinitiation of PEX1,2
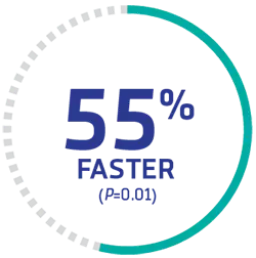
Patients achieved normal platelet count significantly faster with CABLIVI‖
The CABLIVI group (72)†† reached platelet count normalization‖ significantly faster than the PEX and immunosuppressive therapy group (73).
.png)
SIGNIFICANTLY FASTER
time to platelet normalization‖ with CABLIVI
Time to platelet count response
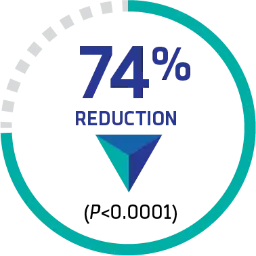
CABLIVI significantly reduced potentially fatal and serious aTTP/iTTP-related events
Compared with PEX and immunosuppressive therapy alone (73), the CABLIVI group (72) demonstrated a significant reduction in a composite endpoint of aTTP/iTTP-related events (36 [49.3%] vs 9 [12.7%], respectively):
.png)
74% REDUCTION IN aTTP/iTTP-RELATED EVENTS
Total composite endpoint of aTTP/iTTP-related events during the study-drug period
| CABLIVI + PEX + Immunosuppressive Therapy | Placebo + PEX + Immunosuppressive Therapy | |
| N=72, n (%)†† | N=73, n (%) | |
| aTTP/iTTP-related death | 0 | 3 (4.1%) |
| Recurrence during treatment§ | 3 (4.2%) | 28 (38.4%) |
| ≥1 major thromboembolic event | 6 (8.5%) | 6 (8.2%) |
| Total | 9 (12.7%) | 36 (49.3%) |
Four aTTP/iTTP-related deaths occurred during the trial, including 1 non–treatment-related death in the CABLIVI group during the treatment-free follow-up period and 3 deaths in the placebo group during the treatment period.2
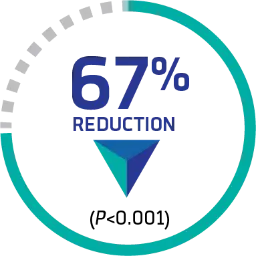
CABLIVI resulted in significantly fewer recurrences requiring reinitiation of PEX§
.png)
67% REDUCTION IN RECURRENCE
during treatment and through 28 days post-treatment vs PEX and immunosuppressive therapy alone 9 (13%) vs 28 (38%), respectively
Incidence of recurrences during treatment and throughout the 28-day follow-up post-treatment
6 patients required reinitiation of PEX after treatment with CABLIVI was stopped
- These patients had ADAMTS13 activity levels <10%, indicating the underlying disease was still active when treatment was stopped
Suppressed ADAMTS13 activity may signify the need to extend CABLIVI treatment
§Thrombocytopenia after initial recovery of platelet count (platelet count ≥150,000/μL) that required reinitiation of daily PEX was considered a recurrence. Recurrences were termed exacerbations if they occurred within 30 days of the last PEX and relapses if they occurred more than 30 days after the last PEX.2
‖Platelet count normalization was defined as platelet count ≥150,000/μL with discontinuation of daily PEX 5 days thereafter.2
††71 patients received at least 1 dose of study drug.
Healthcare resource utilization
In the HERCULES study, lower HRU was associated with early CABLIVI use vs placebo2,4,5
Mean days |
Mean days |
Mean days |
Mean volume | |
|---|---|---|---|---|
CABLIVI + PEX + IS |
3.4 |
9.9 |
5.8 |
21.3 L |
Placebo + PEX + IS |
9.7 |
14.4 |
9.4 |
35.9 L |
HRU Cost Differential |
6.3 |
4.5 |
3.6 |
14.6 L |
These data were collected prospectively. Descriptive statistics were run but were not tested for significance. The clinical significance of these data is unknown.
To monetize the healthcare resource use outlines above, the 2019/20/21 MEDPAR file (100% sample) was used to obtain Medicare payments and charges for aTTP/iTTP cases with Medicare FFS coverage. The Medicare payment amount reflects the base DRG and outlier payments. A standard cost to charge ratio was applied to generate an estimated cost (ie, the cost to the hospital to deliver care) for ICU and non-ICU days—the latter factoring in patients who spend a portion of their time in the general ward and those who spend their entire stay in the general ward. The cost of PEX is based on Heatwole et al. Mean days in the ICU, cost per day: $2,702; mean days in the hospital, cost per day: $1,795–$2,193; mean days receiving PEX, cost per day: $4,597.
In HERCULES, patients who received CABLIVI needed an average of 5.8 days of PEX treatment, compared with an average of 9.4 days of PEX treatment in the placebo group.2
Safety
The safety of CABLIVI was established in 106 patients across 2 studies1‡‡
Overall bleeding events
58%
CABLIVI group
vs
43%
PEX + immunosuppressive therapy alone
Severe bleeding was reported in 1% of patients for each of the following events:
Most frequent adverse reactions in phase 2 and phase 3 clinical studies1
| CABLIVI + PEX + Immunosuppressive therapy N=106, n (%) | PEX + Immunosuppressive therapy N=110, n (%) | ||
| GI disorders | Gingival bleeding | 17 (16) | 3 (3) |
| Rectal hemorrhage | 4 (4) | 0 (0) | |
| Abdominal wall hematoma | 3 (3) | 1 (1) | |
| General disorders and administration site conditions | Fatigue | 16 (15) | 10 (9) |
| Pyrexia | 14 (13) | 12 (11) | |
| Injection site hemorrhage | 6 (6) | 1 (1) | |
| Catheter site hemorrhage | 6 (6) | 5 (5) | |
| Injection site pruritus | 3 (3) | 0 (0) | |
| Musculoskeletal and connective tissue disorders | Back pain | 7 (7) | 4 (4) |
| Myalgia | 6 (6) | 2 (2) | |
| Nervous system disorders | Headache | 22 (21) | 15 (14) |
| Paresthesia | 13 (12) | 11 (10) | |
| Renal and urinary disorders | Urinary tract infection | 6 (6) | 4 (4) |
| Hematuria | 4 (4) | 3 (3) | |
| Reproductive system and breast disorders | Vaginal hemorrhage | 5 (5) | 2 (2) |
| Menorrhagia | 4 (4) | 1 (1) | |
| Respiratory, thoracic, and mediastinal disorders | Epistaxis | 31 (29) | 6 (6) |
| Dyspnea | 10 (9) | 5 (5) | |
| Skin and subcutaneous tissue disorders | Urticaria§§ | 15 (14) | 7 (6) |
‡‡Adverse reactions reported in ≥2% of patients treated with CABLIVI and that occurred more frequently than placebo during the blinded periods of the phase 2 and phase 3 studies.
§§Urticaria was seen during PEX.
Seven patients (7%) in the CABLIVI group experienced an adverse reaction leading to study drug discontinuation. None of the adverse reactions leading to discontinuation were observed in more than 1% of patients.1
Pediatric population
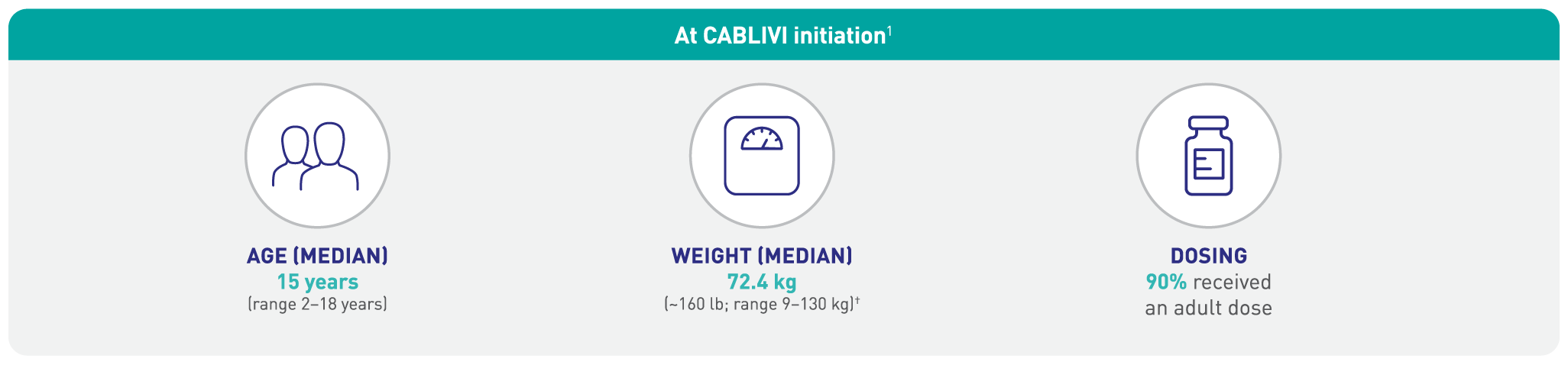
Efficacy of CABLIVI* was established in pediatric patients ≥12 years old with aTTP/iTTP1
Study design: An observational, retrospective chart review was conducted to evaluate the efficacy of CABLIVI in 30 pediatric patients (≤18 years of age) diagnosed with aTTP. At the age of aTTP diagnosis, 21 patients (70%) were >12 years of age and 9 patients (30%) were ≤12 years old. The median study duration was 113 days (~3.7 months). Among the 30 patients, 29 received PEX for a median of 6 days after CABLIVI initiation. Major efficacy outcomes included clinical remission, time to platelet count response, refractory disease, and disease recurrence.1
*In combination with PEX and immunosuppressive therapy.
CABLIVI is used in combination with PEX and immunosuppressive therapy to treat pediatric patients with aTTP/iTTP
.png)
†1 kilogram=2.2 lb; 72.4 kg x 2.2 lb=159.3 lb.
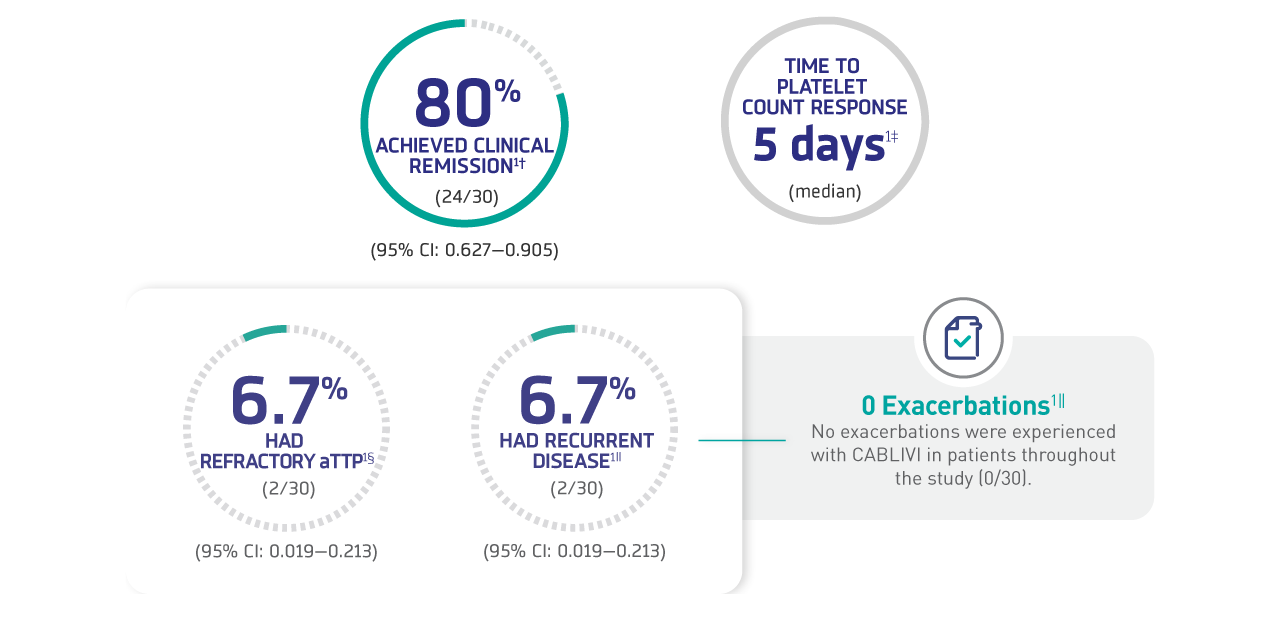
Major efficacy outcomes1*
*In combination with PEX and immunosuppressive therapy.
†Proportion of patients achieving clinical remission, defined as platelet count remaining ≥150×109/L and an LDH level <1.5×ULN for ≥30 days after cessation of TPE.
‡Time to platelet count response, defined as time from caplacizumab initiation to initial platelet count ≥150 x 109/L with subsequent stop of daily TPE within 5 days.
§Proportion of patients with refractory iTTP, defined as lack of doubling of platelet count after 4 days of caplacizumab treatment and LDH level >ULN range.
∥Proportion of patients with recurrent disease (aTTP exacerbation, defined as recurrence ≤30 days after last TPE, or relapse, defined as recurrence >30 days after last TPE).
The adverse reaction profile in pediatric patients ≥12 years was consistent with that in adults:
- The most commonly reported adverse events were epistaxis (n=4; 13.3%) and tachycardia (n=4; 13.3%)
- One serious bleeding adverse reaction was reported (hemorrhage urinary tract)
The safety and effectiveness of CABLIVI in pediatric patients ≥12 years of age with aTTP/iTTP have been established.1*
Enroll patients in HemAssist™ Sanofi Support to give eligible patients access to educational resources and financial assistance
Learn how to access CABLIVI
ADAMTS13=a disintegrin and metalloproteinase with a thrombospondin type 1 motif, 13; CI=confidence interval; DRG=diagnosis-related group; FFS=fee for service; GI=gastrointestinal; HRU=healthcare resource utilization; ICU=intensive care unit; IS=immunosuppressive therapy; ISTH=International Society on Thrombosis and Haemostasis; PEX=plasma exchange; TE=thromboembolic; TTP=thrombotic thrombocytopenic purpura.
INDICATION
References: 1. CABLIVI. Prescribing information. Sanofi. 2. Scully M, Cataland SR, Peyvandi F, et al; HERCULES Investigators. Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2019;380(4):335-346. 3. Zheng XL, Vesely SK, Cataland SR, et al. ISTH guidelines for the diagnosis of thrombotic thrombocytopenic purpura. J Thromb Haemost. 2020;18(10):2486-2495 and suppl/protocol. doi:10.1111/jth.15006 4. Estimated 2019/2020/2021 MEDPAR file. 5. Heatwole C, Johnson N, Holloway R, Noyes K. Plasma exchange versus intravenous immunoglobulin for myasthenia gravis crisis: an acute hospital cost comparison study. J Clin Neuromuscul Dis. 2011;13(2):85-94. doi:10.1097/CND.0b013e31822c34dd
