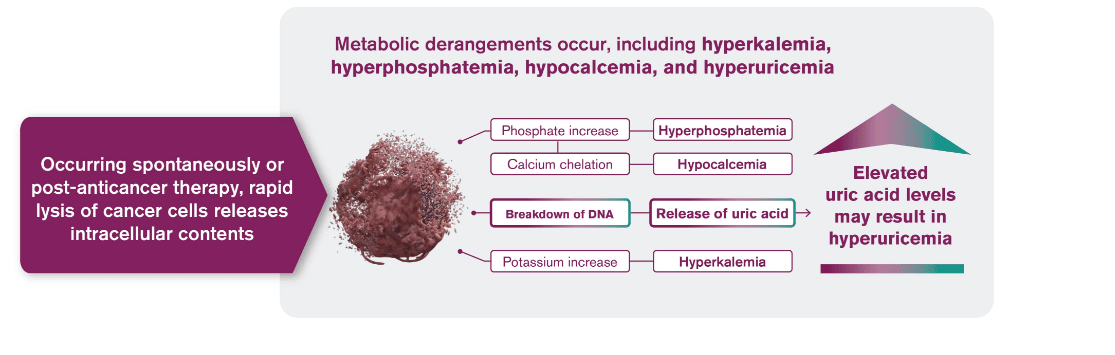
Tumor lysis syndrome (TLS) is caused by a massive release of intracellular contents into peripheral blood that results in metabolic derangements1
Hyperuricemia
Hyperkalemia
Hyperphosphatemia
Hypocalcemia
TLS is prevalent in hematologic malignancies with1:
- High proliferative rate
- Large cellular burden
- High sensitivity to chemotherapy or cytolytic antibody therapy
TLS occurs spontaneously or in response to anticancer therapy, usually 12 to 72 hours after the start of therapy1,2
TLS associated with hyperuricemia may lead to serious clinical complications2
Are your patients at higher risk for TLS than you think?
Use this Risk Assessment Tool to help you identify patients who may be at risk for TLS
VISIT NOW
Highly effective anticancer therapies can promote rapid cell death leading to TLS1-3
- Rapid cell death can cause the release and catabolism of nucleic acids, resulting in the rise of uric acid levels
- Hyperuricemia is one of several metabolic disorders that can lead to TLS, the most common disease-related emergency in hematologic cancers
.png)
Both laboratory and clinical symptoms are key to identifying patients with TLS
Cairo-Bishop classification of TLS4
| LABORATORY TLS |
|
A patient with 2 or more of the following abnormalities within 3 days before to 7 days after initiation of cancer treatment:
|
| CLINICAL TLS |
|
A patient with laboratory TLS and at least 1 of the following:
|
TLS is the most common disease-related emergency in hematologic cancers3
Incidence of TLS in patients with hematologic malignancies5
In a retrospective analysis, a total of 15,051 cases of TLS were identified among the 40,494 patients with hematologic malignancies during this period
- This is based on data from the National Inpatient Sample Database, the largest publicly available, all-payer inpatient database5
|
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) recommend that to best manage TLS, anticipate it and initiate treatment prior to anticancer therapy for patients with CLL/SLL, B-cell lymphoma, AML, and ALL2,6-8 |
ALL=acute lymphoblastic leukemia; AML= acute myeloid leukemia; CLL=chronic lymphocytic leukemia; NCCN=National Comprehensive Cancer Network; SLL=small lymphocytic lymphoma.
References: 1. Cortes J, Moore JO, Maziarz RT, et al. Control of plasma uric acid in adults at risk for tumor lysis syndrome: efficacy and safety of rasburicase alone and rasburicase followed by allopurinol compared with allopurinol alone—results of a multicenter phase III study. J Clin Oncol. 2010;28(27):4207-4213. 2. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for B-Cell Lymphomas. V.2.2025. ©National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed March 24, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org . NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. 3. Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364(19):1844-1854. 4. Edeani A, Shirali A. Chapter 4: Tumor Lysis Syndrome. Onco-Nephrology Curriculum. American Society of Nephrology. 2016. 5. Pathak R, Giri S, Aryal M. Recent trends in the incidence and outcomes of tumor lysis syndrome in hematological malignancies: Data from 2010-2014 National Inpatient Sample. Blood. 2017;130:3390. 6. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. V.2.2025. ©National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed March 24, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org . NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. 7. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Acute Lymphoblastic Leukemia. V.3.2024. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed March 24, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org . NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. 8. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Acute Myeloid Leukemia. V.2.2025. ©National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed March 24, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org . NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.