- Article
- Source: Campus Sanofi
- May 20, 2025
BIOCHEMICAL DIAGNOSTIC STRATEGY IN FEMALES SUSPECTED OF FABRY DISEASE
_430x268.jpg)
Study objective and method
To demonstrate
.png)
The benefits of adding lyso-GL-3 to ►
.png)
Primary diagnostic for testing for ►
.png)
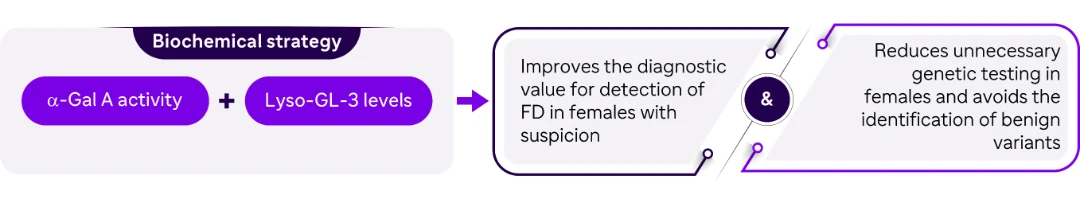
Improving detection rate of potential females with FD
.png)
Avoiding unnecessary genetic testing
|
First large prospective study |
.png)
11,948 females with suspicion of FD
tested for
.png)
Biochemical tests
- α-Gal A activity
- Lyso-GL-3 levels
followed by
.png)
Genetic confirmatory testing in 883 females
.png)
Groups | 01. Low α-Gal A activity High lyso-GL-3 | 02. Low α-Gal A activity Normal lyso-GL-3 | 03. Normal α-Gal A activity High lyso-GL-3 | 04. Normal α-Gal A activity Normal lyso-GL-3 |
| Classical FD very likely (n=61) | Classical FD unlikely (n=184) |
FD very likely (n=256) |
95.8 had normal results (n=11,447*) | |
| Genetic confirmatory test | No further testing required | |||
Results
.png)
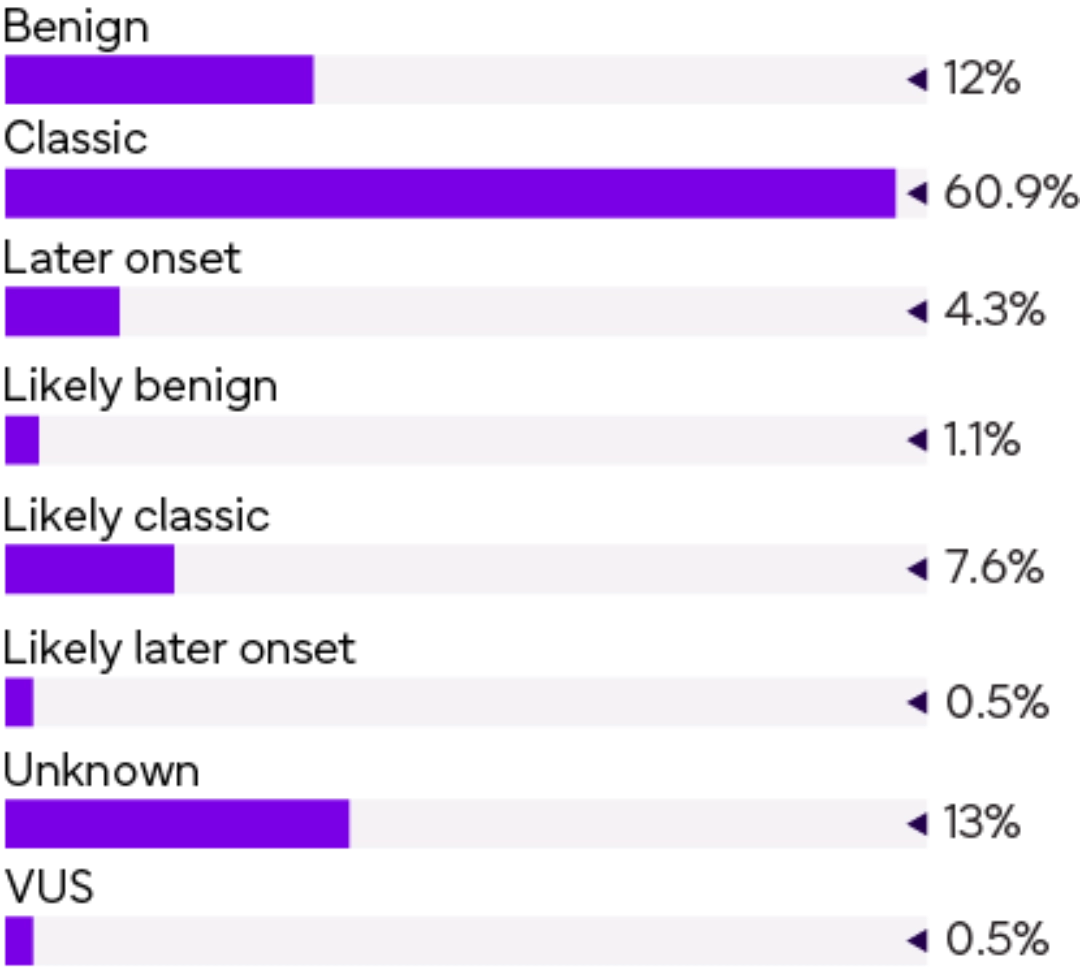
(184/883) females were identified with one or two GLA variants.
| Distribution of genetically positive samples |

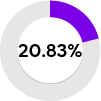
Better indicator of FD
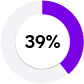
Elevated lyso-GL-3 levels (39% PPV)
.png)
Low α-Gal A activity (6% PPV)
Clearly negative results for both biochemical parameters:
Unlikely to have FD, even in clinically highly suspicious cases
| Proposed diagnosis decision tree |
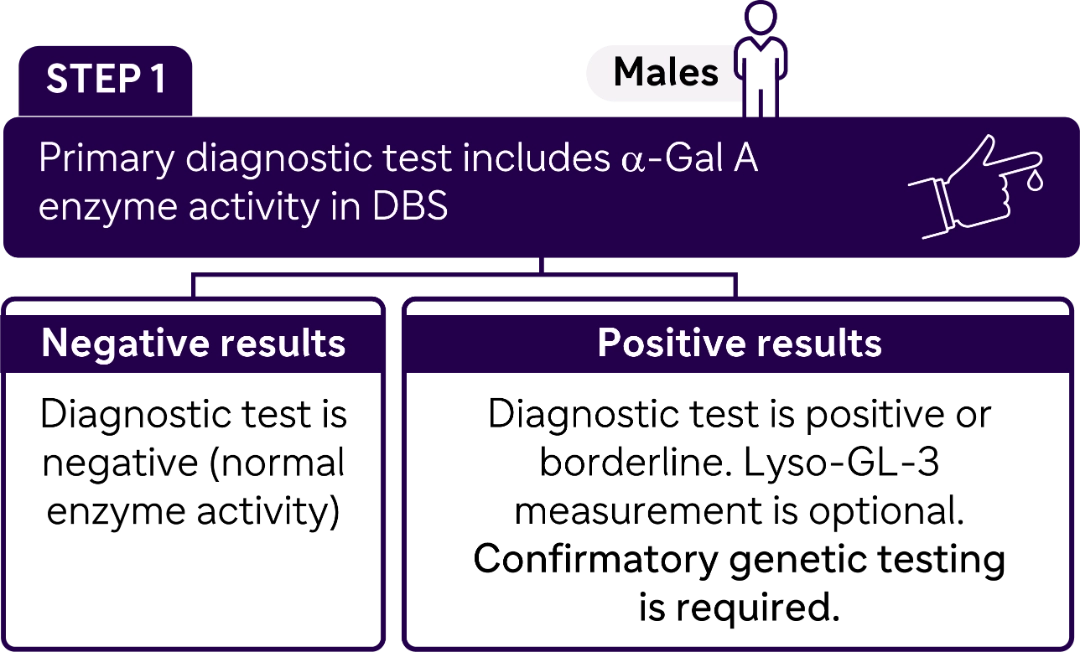
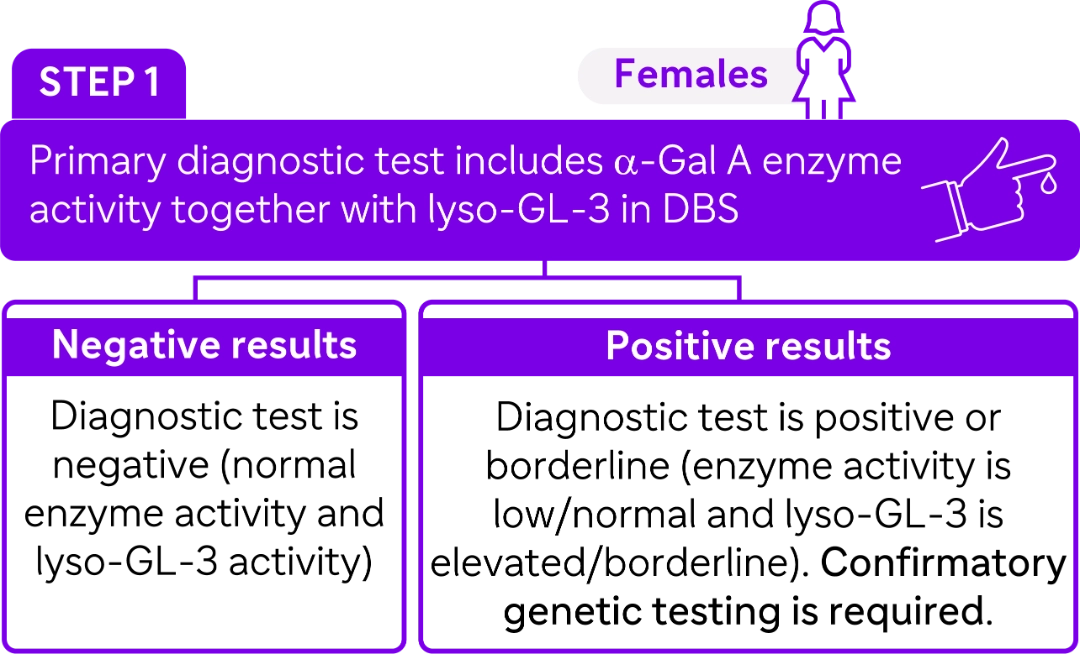
STEP 2
Once enzyme deficiency is confirmed, molecular testing is performed to identify GLA genetic variants.
STEP 2
Once enzyme activity (deficient or not) together with elevated biomarker is confirmed, molecular testing is performed to identify GLA genetic variants.
Conclusion
*Mutation analysis performed in 389 cases.
α-Gal A: Alpha-galactosidase A; dbFGP: Fabry disease genotype–phenotype database; FD: Fabry disease; Lyso-GL-3: Globotriaosylsphingosine; PPV: Positive predictive value; DBS: Dried blood spotting; GLA: Galactosidase alpha; VUS: Variant of unknown significance.
MAT-BH-2400131-V1-March 2024