- Article
- Source: Campus Sanofi
- Sep 4, 2025
Nirsevimab RWD Metanalysis

Systematic review and meta-analysis studies across five countries1
32 cohort and case-control studies were included in a systematic review, of which 27 were included in the meta-analysis† , comparing nirsevimab and control groups during the 2023-2024 RSV season. Most studies were conducted in inpatient care settings, followed by outpatient settings and emergency rooms.

France
8 studies

Spain
18 studies

USA
4 studies

Italy
1 study

Luxembourg
1 study
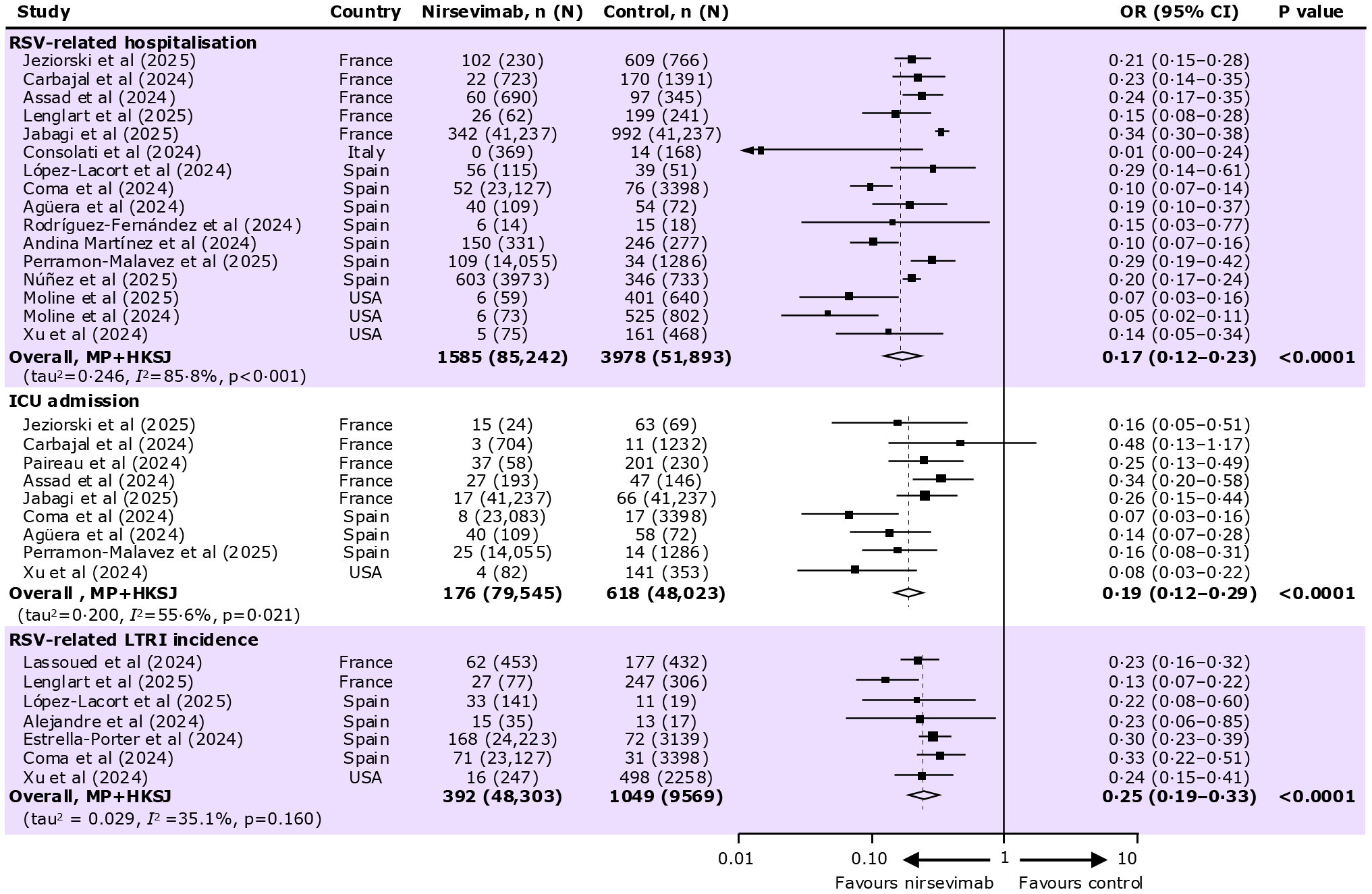
RSV related hospitalization

- Overall, 16 studies reported data on RSV-related hospitalizations in infants aged 0–12 months
- RSV-related hospitalizations were observed in:
- Nirsevimab group: 1,585 / 85,242 infants
- Control group: 3,978 / 51,893 infants

- In infants 0-12 months, nirsevimab administration is associated with an 83% (OR: 0.17, 95% CI: 0.12–0.23, p<0.0001) reduction in the risk of RSV-related hospitalization
ICU admission

- Overall, 9 studies reported data on RSV-related ICU admissions in infants
- ICU admissions were observed in:
- Nirsevimab group: 176 / 79,545 infants
- Control group: 618 / 48,023 infants
- Nirsevimab administration is associated with an 81% (OR: 0.19, 95% CI: 0.12–0.29, p<0.0001) reduction in the risk of RSV-related ICU admissions
RSV-related LRTI incidence

- Overall, 7 studies reported data on RSV-related LRTI incidence in infants.
- RSV-related LRTI were observed in:
- Nirsevimab group: 392 / 48,303 infants
- Control group: 1,049 / 9,569 infants
- Nirsevimab administration is associated with an 75% (OR: 0.25, 95% CI: 0.19–0.33, p<0.0001) reduction in the risk of RSV-related LRTI incidence
Limitations

- Observational studies may include bias from confounding factors
- Subgroup analyses on gestational age or pre-existing conditions were not performed due to insufficient data
- The effect of nirsevimab on specific outcomes, such as bronchiolitis, was not assessed
- Prioritization and distribution of nirsevimab across regions can limit the generalizability of the findings
- Previous RSV exposure might have influenced the estimates of nirsevimab effectiveness during the 2023-2024 season
- Differences in baseline characteristics can introduce variability in outcomes

CONCLUSION
Real world evidence consistently shows a significant reduction in RSV-related outcomes among nirsevimab recipients across studies and countries. These findings underscore the importance of infant immunization in alleviating the burden of RSV disease in infants
Nirsevimab demonstrated public health impact
NIRSE-GAL (Spain)3
Reduction of RSV-related LRTI hospitalization in Galicia compared to previous season*
Reduction of RSV-related LRTI hospitalization in Galicia compared to previous season*
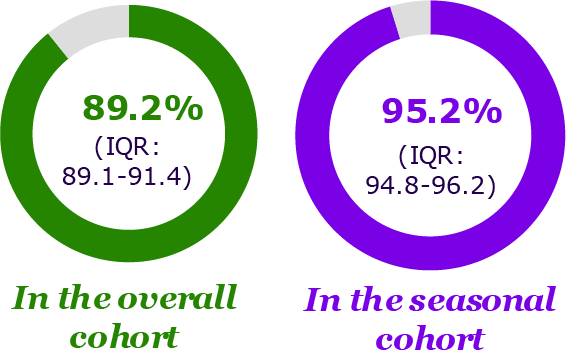
* Median of RSV-related LRTI reduction compared to 2016-2017, 2017-2018, 2018-2019, 2019-2020 and 2022-2023 seasons
- To evaluate the effectiveness and impact of nirsevimab in Galicia, Spain
- Coverage rate: 92% (13,320) of 14,476 eligible infants received nirsevimab
- In the catch-up cohort (i.e., born in the period April 1 to Sept 24, 2023), 88.5% (6249) of 7062 eligible infants received nirsevimab
- In the seasonal cohort (i.e., born in the period Sept 25, 2023, to March 31, 2024), 95.3% (7071) of 7414 eligible infants received nirsevimab
- 96.6% (348) of 360 additional infants classified as high-risk were immunized
Nirsevimab was:
- 80.3% effective as reducing RSV-related LRTI hospitalization with oxygen support (95%CI: 54.6-91.5)
- 70.7% effective at reducing RSV-related LRTI hospitalization (95%CI: 42.4-85.1)
- 46.0% effective at reducing all-cause bronchiolitis or bronchitis hospitalization (95%CI: 6.8-68.7)
- 35.2% effective at reducing all-cause LRTI hospitalization (95%CI: - 3.8-59.6)
Nirsevimab significantly reduced infant hospitalization for RSV-related LRTI and RSV-related LRTI requiring oxygen compared to previous season
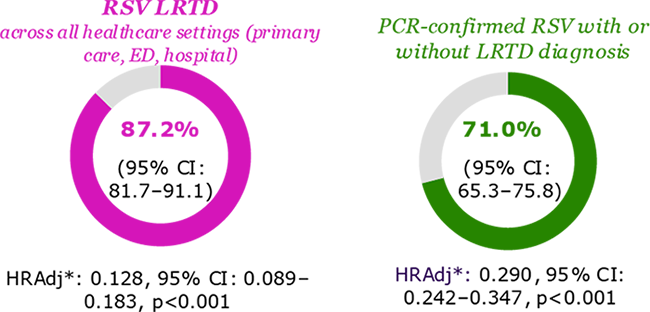
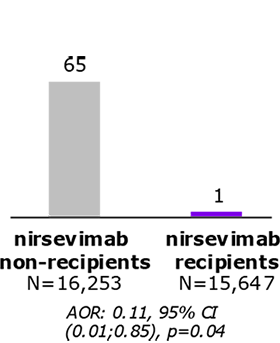
BEAR: Beyfortus Effectiveness Against Medical Attended RSV Events in Infants (USA)4
- 15,647 out of 31,900 included infants received nirsevimab
- RSV-LRTD were observed in:
- 35 infants from nirsevimab group (6.1/1000 PY)
- 462 infants from untreated infants (58.5/1000 PY)
Nirsevimab demonstrated effectiveness against …
*Nearly half of eligible KPNC healthy infants born at term received nirsevimab during the 2023–2024 season
Incidence of hospitalization during RSV LRTD episode
Mean number of medical encounters during RSV LRTD episode
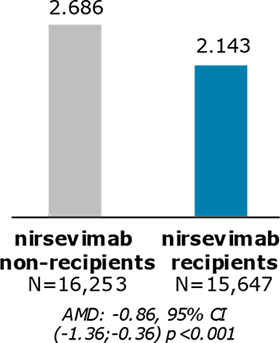
Among nirsevimab recipients who did experience an RSV LRTD episode, the mean number of medical encounters was significantly lower than among infants who had not received RSV prevention
Nearly half of eligible healthy term infants received nirsevimab during the 2023-2024 season. Compared with non-immunized infants, those who had RSV LRTD after nirsevimab had significantly fewer medical encounters and were less likely to be hospitalized.
NIRSE-CL (Chile)5
- Coverage: 97.5% of the new-born group and 90.4% of the infant group have been immunized
|
Eligible population |
Non-eligible population | |
| Cumulative hospitalization Compared to previous years hospitalizations |
1,211 |
2,892 |
| Hospitalization in CCU Compared to previous years CCU hospitalization |
249 |
681 |
- In the eligible population, hospitalizations due to RSV in 2024 were significantly lower than those of previous years
- In the eligible population, RSV hospitalizations in CCUs in 2024 were significantly lower than those of previous years
- The mean length of stay for RSV hospitalization is significantly lower in the immunized group (4.19 days) vs the non-immunized group (5.25 days)
Nirsevimab was well accepted by parents in Chile and was associated with a significant reduction in the overall public health burden of RSV disease in infants compared to previous years
†3 studies from Spain, 1 from France and 1 from Luxembourg were not included in the meta-analysis as they compared the nirsevimab and control groups over two different RSV seasons (2022-2023 and 2023-2024)
AMD, adjusted difference in means; AOR, adjusted odds ratio; BEAR, Beyfortus effectiveness against medical attended RSV events in infants; CCU, critical care unit; CI, confidence interval; HRAdj, adjusted hazard ratio; ICU, intensive care unit; LRTD, lower respiratory tract disease; LRTI, lower respiratory tract infection; OR, odds ratio; PY: Person-Years; RSV, Respiratory syncytial virus USA, United States of America.
- Sumsuzzman, et al. Lancet Child Adolesc Health.2025;9: 393–403
- Turzalde-Mapili MWR, et al. Front Pediatr. 2023;11:1132740.
- Mallah N, et al. Lancet Infect Dis. 2025;25(2):e62-e63.
- Hsiao A, et al. Ann Allergy Asthma Immunol. 2024;133(6 Suppl 2):S3-S4.
- NirseCL: Monitoring the impact of Nirsevimab in the winter campaign in 2024 in Chile. Available at: https://nirse.isci.cl/#reporte. Accessed May 2025.
MAT-BH-2500380 v1.0 | July 2025