- Article
- Source: Campus Sanofi
- 24 Feb 2025
An Introduction to Severe Asthma
What is Severe Asthma?
Severe asthma refers to asthma which does not get better with the usual treatment leaving patients with breathing problems most of the time, meaning that a different treatment approach may be necessary to control the symptoms 1,2
- ~200,000 people in the UK have severe asthma*1,2
- 39% (61/155) of people that died from asthma were reported to have severe asthma between 2012–2013†3
- In a UK study‡,~93% (748/808) of severe asthma patients had a least one corticosteroid-related morbidity4
*Approximately 4% of the 5.4 million people in the UK who have asthma have severe asthma (approximately 200,000–250,000). GINA defines severe asthma as asthma that remains uncontrolled despite optimised treatment with high-dose ICS-LABA or that requires high-dose ICS-LABA to prevent it from becoming uncontrolled1,2,5
†In the National Review of Asthma Deaths (NRAD) (2014) sufficient data was available on 195 people who died of asthma between 2012–2013. From 195 people, 155 had their asthma severity recorded prior to death. No history of asthma was reported as 3% (4/144), mild asthma was reported as 9% (14/155), moderate asthma was reported as 49% (76/155) and severe asthma was reported as 39% (61/155). The NRAD is the first UK-wide investigation into asthma deaths that took place between Feb 2012 to Jan 2013. Experts such as general practitioners (GPs), nurses and pharmacists looked at medical records and other information relating to these deaths from doctors’ surgeries, hospitals, emergency services and coroners’ offices. Severity was defined as follows: The BTS/Scottish Intercollegiate Guidelines Network (SIGN) treatment steps 1 and 2 were used as a surrogate for mild and moderate severity; those who were prescribed four asthma medications and those who had been admitted to hospital in the past year, needed OCS daily or had two or more prescriptions for systemic corticosteroids in the past year were classified as severe.3
‡A cross-sectional observational study to determine the prevalence of systemic corticosteroid-induced morbidity in severe asthma using the Optimum Patient Care Research Database (OPCRD), (7,195 subjects in three age- and gender-matched groups) - severe asthma (GINA treatment step 5 with four or more prescriptions/year of OCS, N=808), 770 subjects with severe asthma from the BTS Difficult Asthma Registry (442 receiving daily OCS to maintain disease control). Severe asthma was defined by the Global Initiative for Asthma (GINA) treatment step 5 with four or more prescriptions/year of OCS. 770 subjects with severe asthma from the BTS Difficult Asthma Registry (442 receiving daily OCS to maintain disease control).4
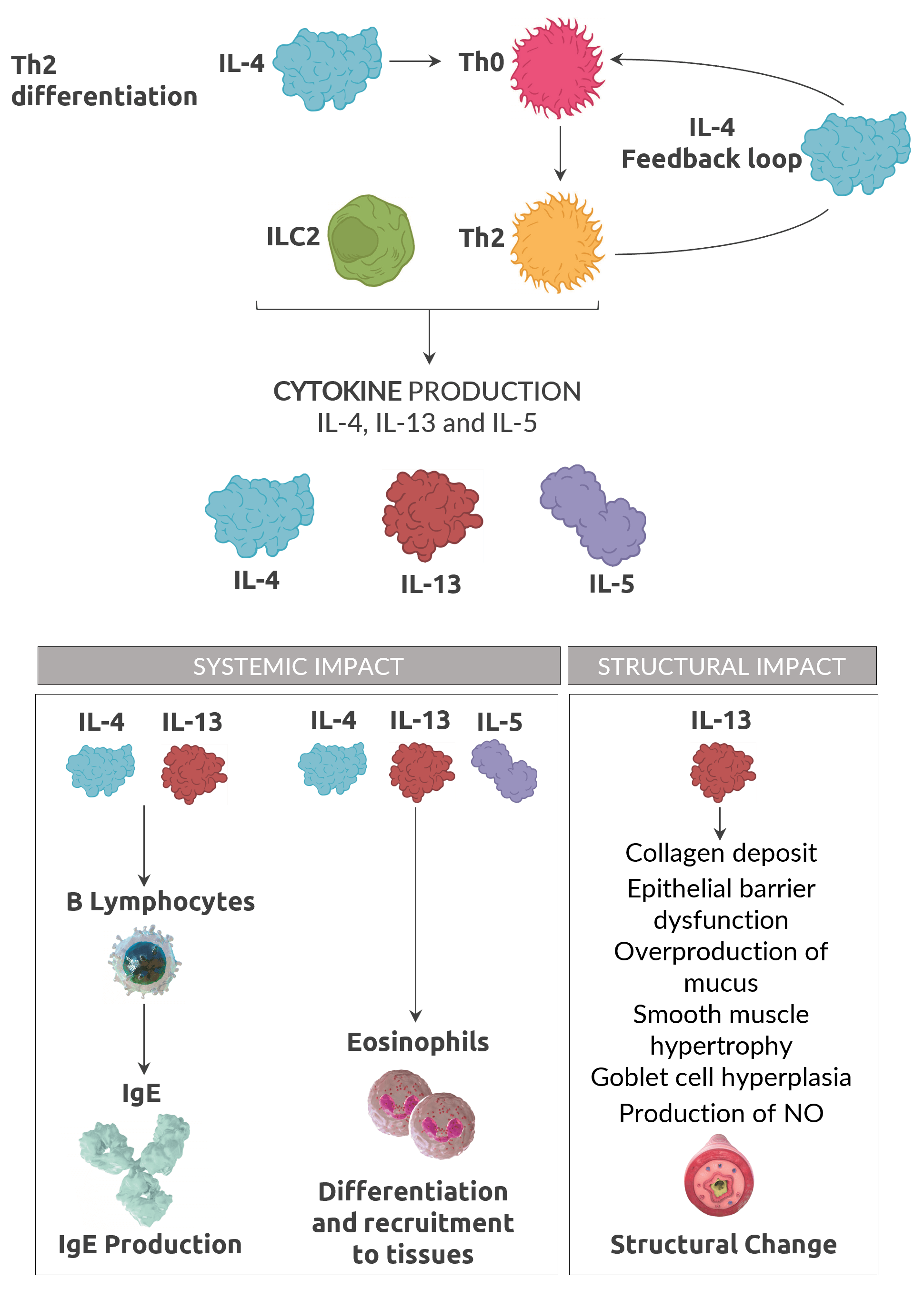
Type 2 Inflammation
No single biomarker fully captures the complexity of Type 2 inflammation in asthma6,7
50 - 70% of adult asthma patients have Type 2 Inflammation, with close to 1 in 2 patients presenting with multiple Type 2 inflamatory biomarkers7,8,9.
%20(1).13633060878149093984.png)
Adapted from Robinson 201710 and Gandhi et al. 201611
Biomarkers
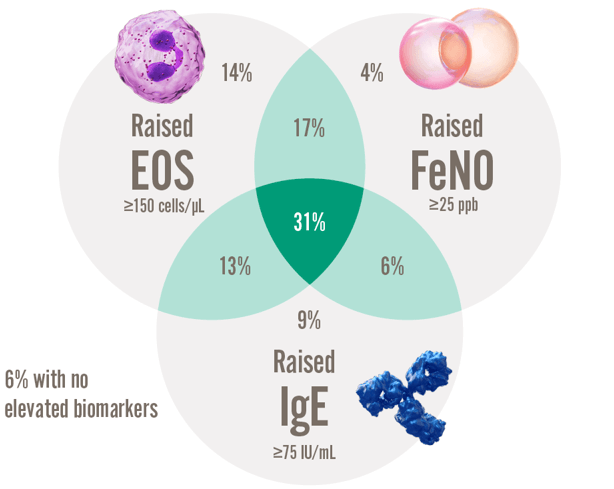
59% of patients with severe asthma have several elevated biomarkers12
Type 2 biomarkers support the assessment of type 2 inflammation10,11, 13
- Maturation and differentiation driven by IL5, trafficking to airway driven by IL-4 and IL-13
- Involved in maintaining long-term inflammation
- Associated with fixed airway obstruction
- Secretion induced by IL-4 and IL-13
- Associated with allergy
- Indicator of IL-13 driven inflammation
- Production induced by IL-4 and IL-13
- Indicator of corticosteroid responsiveness
- Useful in assessing asthma severity
%20(1).7220821255109089383.png)
% of patients presenting with each kind of raised biomarker12.
Elevated EOS, FeNO, OR IgE is evidence of Type 2 Asthma5
88% of patients with severe asthma may have type 2 inflammation15
DUPIXENT (dupilumab) is shown to work in patients with severe asthma type 2 biomarkers ((EOS ≥ 150 cell/μL and/or FeNO ≥ 25 ppb with or without elevated IgE)5,16
Over HALF of type 2 asthma patients have more than one elevated type 2 biomarker12,§
- Elevated EOS and FeNO are strong predictors of a good asthma response to DUPIXENT16
- Oral Corticosteriod (OCS) may suppress type 2 biomarkers, which should be accounted for when assessing type 2 asthma status in patients taking OCS 5,17.
§ Within the International Severe Asthma Registry (ISAR), data were analysed in adults with severe asthma with available biomarkers (n = 1,175) from 10 countries in North America, Europe, and Asia, with respecified thresholds for biomarker positivity - (serum IgE ≥ 75 IU/mL, blood eosinophils ≥ 300 cells/mL, and FeNO ≥ 25 ppb); and with hierarchical cluster analysis using biomarkers as continuous variables. Distinct clusters were shown with significant overlap of biomarker positivity in severe asthma.12
Watch this video to learn more about Type 2 inflammation in asthma
Guidelines
GINA recommends assessing the severe asthma phenotype during high-dose inhaled corticosteroid (ICS) or daily OCS treatment for evidence of Type 2 inflammation, including:
- Blood eosinophils (EOS) ≥ 150 cells/µL
- Asthma that is clinically allergen driven
- Fractional exhaled nitric oxide (FeNO) ≥ 20 ppb
- Need for maintenance OCS
- Sputum EOS ≥ 2%
Full GINA guidance available at: https://ginasthma.org/2024-report/
BTS/SIGN recommend the options for investigation of eosinophilic inflammation or atopy are:
- FeNO
- Blood EOS
- Skin-pick test, IgE
Full BTS/SIGN guidance available at: https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma.
1 Asthma UK. What is severe asthma? Available at: https://www.asthmaandlung.org.uk/conditions/severe-asthma/what-severe-asthma. Date last accessed: February 2025.
2 Asthma UK. Do no harm: safer and better treatment options for people with asthma. Available at: https://www.asthmaandlung.org.uk/sites/default/files/2023-03/severe-asthma_report_final.pdf. Date last accessed: February 2025.
3 Royal College of Physicians. Why Asthma still kills: The National Review of Asthma Deaths (NRAD). Available at: https://www.rcp.ac.uk/improving-care/resources/why-asthma-still-kills/. Date last accessed: February 2025.
4 Sweeney J, et al. Thorax. 2016;71(4):339–346.
5 Global Initiative for Asthma. Global strategy for asthma management and prevention, 2024. Available at: https://ginasthma.org/2024-report/. Date last accessed: February 2025.
6 Amaral R, et al. Clin Transl Allergy. 2018;8:13.
7 Seys SF, et al. Respir Res. 2017;18:39.
8 Peters MC, et al. J Allergy Clin Immunol. 2014;133(2):388-394.
9 Tran TN, et al. Ann Allergy Asthma Immnol. 2016(116):37-4.
10 Robinson D, et al. Clin Exp Allergy. 2017;47(2):161–175.
11 Gandhi NA, et al. Nat Rev Drug Discov. 2016;15(1):35–50.
12 Denton E, et al. J Allergy Clin Immunol Pract. 2021;9(7);2680-2688.e7.
13 Alving K et al. Eur Respir Monogr 2010; 49: 1-31.
14 Carr TF, et al. World Allergy Organ J. 2016;9:21.
15 Buhl R, et al. J Allergy Clin Immunol Pract. 2020;8(8):2630-2639.e6.
16Castro M, et al. N Eng J Med. 2018;378:2486–2496.
17Busby X et al. Respiratory Medicine 2020; 173,106156.
18 GINA. Difficult-to-treat & severe asthma in adolescent and adult patients. A GINA Pocket Guide for Health Professionals. Available at: https://ginasthma.org/difficult-to-treat-and-severe-asthma-guide/. Date last accessed: February 2025.
19 BTS and SIGN. SIGN 158. British guideline on the management of asthma. Available at: https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma. Date last accessed: February 2025.
.jpg)
MAT-XU-2400861 (v2.0) Date of Preparation: Februrary 2025