%20(1).jpg)
OPEN UP A WORLD BEYOND THEIR SEVERE ASTHMA SYMPTOMS
DUPIXENT combines the ability to help:
TARGETIL-4 and IL-13, two key and central drivers of type 2 inflammation1-3 |
TREATOCS-dependent patients (Restricted use in SCOTLAND ONLY please see reimbursement section for more information) with type 2 severe asthma4 |
TRANSFORMOver 1 million patients treated worldwide across indications driven by type 2 inflamation5 |
INDICATIONS
DUPIXENT is indicated in patients six years and older as add-on maintenance treatment for severe asthma with type 2 inflammation characterised by:
- raised blood eosinophils (EOS)
- and/or raised fraction of exhaled nitric oxide (FeNO)
- who are inadequately controlled with high-dose (ages twelve and older) or medium-to-high dose (ages six to eleven) inhaled corticosteroids (ICS)
- another medicinal product for maintenance treatment.1
Mechanism of action (MOA)/ mechanism of disease (MOD)
DUPIXENT IS THE ONLY BIOLOGIC THAT DIRECTLY TARGETS INTERLEUKIN (IL)-4 AND IL-13 SIGNALING TO REDUCE TYPE 2 INFLAMMATION IN SEVERE ASTHMA.1-3
Type 2 inflammation leads to structural changes in the airway, a higher risk of exacerbations, and an increased use of oral corticosteroids (OCS).2,6,7

IL-4 and IL-13 are key drivers of type 2 inflammation in severe asthma1-3
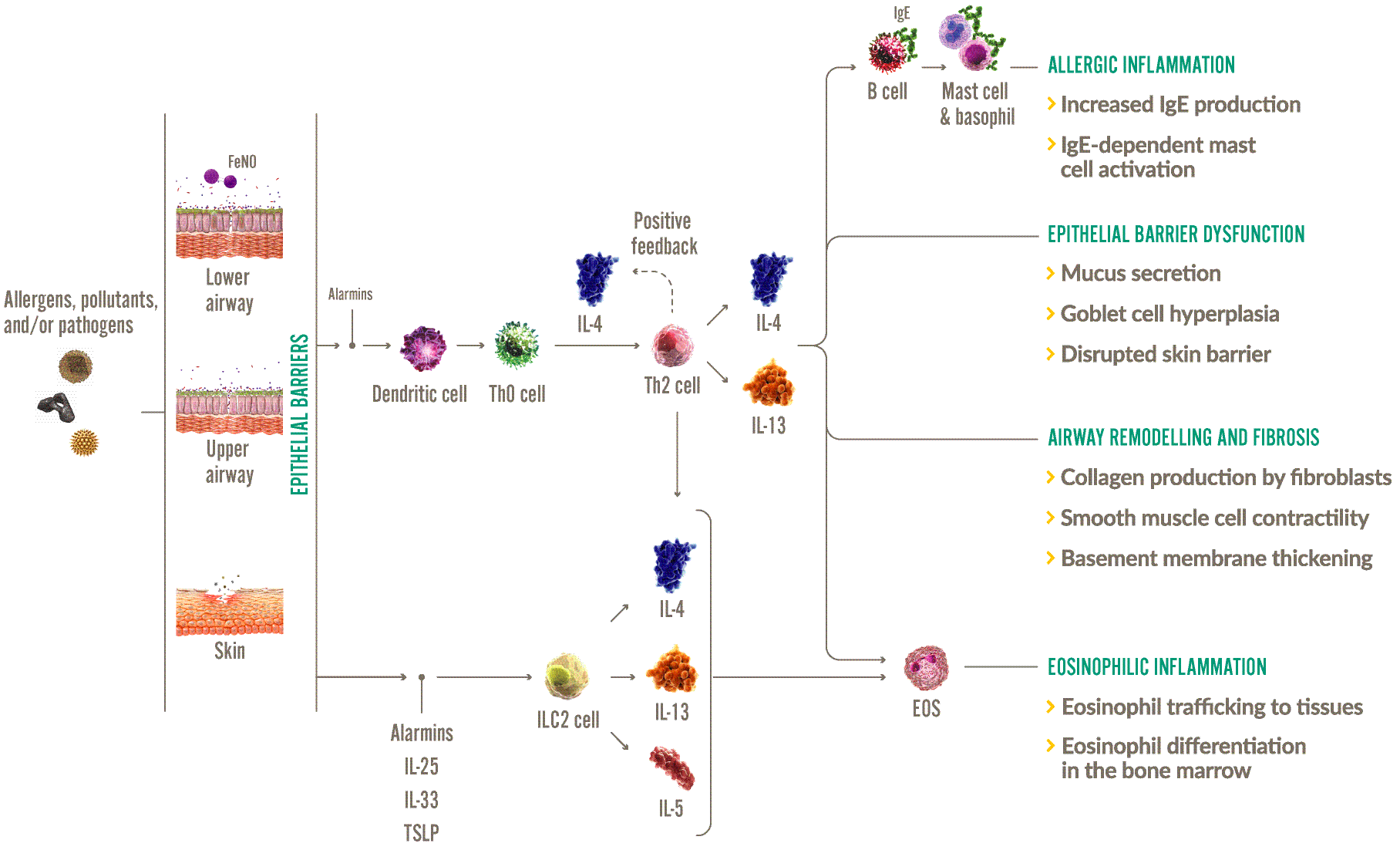
EOS, eosinophils; IgE, immunoglobulin-E; IL, interleukin; ILC2, group 2 innate lymphoid cell; Th, T helper; TSLP, thymic stromal lymphopoietin.
Adapted from: Robinson D, et al. 2017,3 Gandhi NA, et al. 2016,2 Le Floc’h A, et al. 2020.8
| LOCAL INFLAMMATION2,3,9 | SYSTEMIC INFLAMMATION2,3 |
|
Allergic inflammation
Eosinophilic inflammation
IL-4 and IL-13, along with IL-5, play a role in eosinophil trafficking |
Efficacy
DUPIXENT OPENS UP A WORLD FOR A RANGE OF SEVERE ASTHMA PATIENT TYPES
Patient profiles are representative and are not actual DUPIXENT patients.

Type 2 Severe Asthma (12+)
Learn more

Children with Severe Asthma (6-11)
Learn more

OCS-dependent Patient with Severe Asthma (12+) SCOTLAND ONLY
Learn more
Daniel, 45
Adult with Type 2 Severe Asthma patient (12+)

Diagnosed with severe asthma
High dose of ICS, LABA, and LAMA.
Experiences frequent and distressing asthma exacerbations that regularly disrupt his day-to-day life.
Medical history
| Blood EOS levels | 300 cells/μL |
| FeNO | 25 ppb |
| Severe exacerbations in the last year | > 4 |
| Lung function | 70% FEV1 |
ELEVATED EOS, FeNO, OR IgE IS EVIDENCE OF TYPE 2 ASTHMA7
50-70% of people with severe asthma have underlying Type 2 Inflammation10,11
DUPIXENT is shown to work in patients with elevated type 2 biomarkers (EOS ≥150 cells/μL and/or FeNO ≥25 ppb with or without elevated IgE)1,7
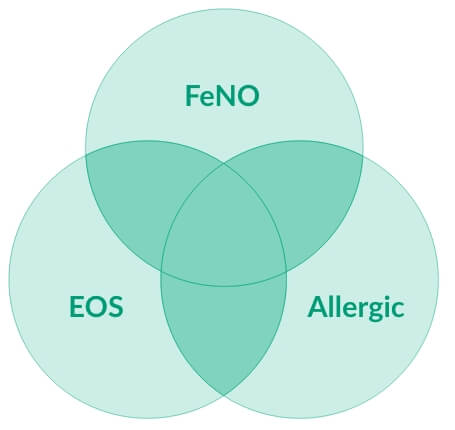
EOS, eosinophils, FeNO, fraction exhaled nitric oxide
OVER HALF (67%) of severe Type 2 asthma patients have more than one elevated Type 2 biomarker12
- Elevated EOS and FeNO are potential predictors of a good asthma response to DUPIXENT7
- Biomarkers of Type 2 inflammation may be suppressed by systemic corticosteroid use; this should be taken into consideration to determine Type 2 status in patients taking OCS7
Quest Trial
Trial design13
- QUEST was a 52-week,* double-blind, placebo-controlled, Phase 3 study in patients with uncontrolled moderate-to-severe asthma and patients were enrolled irrespective of baseline blood EOS counts or other Type 2 biomarkers13
- Clinical trials included patients with uncontrolled moderate-to-severe asthma driven by Type 2 inflammation – study was
designed prior to marketing authorisation. DUPIXENT is indicated in adults and adolescents ≥12 years of age as add-on maintenance treatment for severe asthma with Type 2 inflammation characterised by raised blood EOS and/or raised FeNO, who are inadequately controlled with high-dose ICS plus another medicinal product for maintenance treatment1
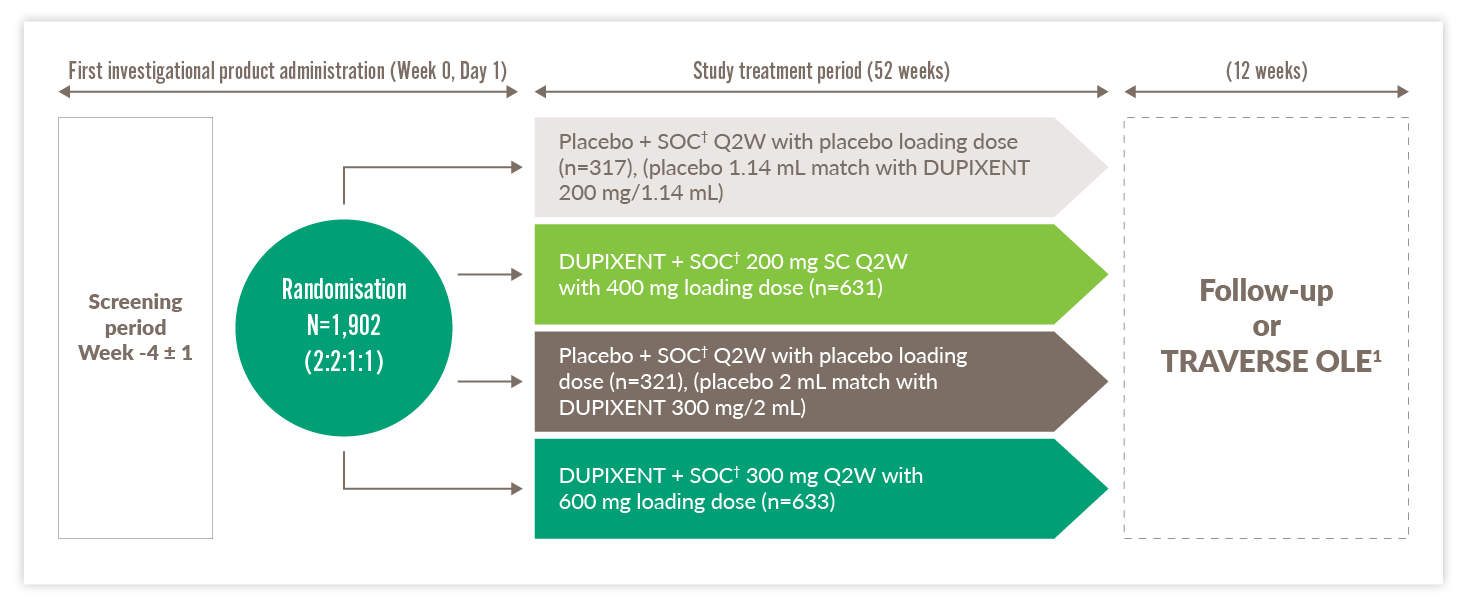
OLE, open-label extension; Q2W, every two weeks; SC, subcutaneous; SOC, standard of care
Primary endpoints13
- Annualised rate of severe exacerbation events over 52 weeks (was met)‡
- Forced expiratory volume in the first second (FEV1) change from baseline at Week 12 (was met)
*QUEST consisted of a 52-week treatment phase, and a 12-week follow-up period.13 †Standard of care (SOC) involved treatment with a medium-to-high-dose inhaled glucocorticoid (fluticasone propionate at a total daily dose of ≥500 μg or equipotent equivalent) plus up to two additional controllers (e.g., a long-acting beta antagonist (LABA) or leukotriene receptor antagonists (LTRA)).13 ‡A severe exacerbation was defined as a deterioration of asthma requiring: use of systemic corticosteroids for ≥3 days; or hospitalisation or emergency room visit because of asthma, requiring systemic corticosteroids. Annualised event rate was the total number of exacerbations that occurred during the treatment period divided by the total number of participant years treated.13
QUEST TRIAL POST HOC RESULTS
Least squares mean (LSM) change from baseline in 5-item Asthma Control Questionnaire (ACQ-5) scores at Week 5214
- Presented below is the post hoc analysis of patients with EOS ≥150 cells/μL and FeNO ≥25 ppb and high-dose ICS at baseline14
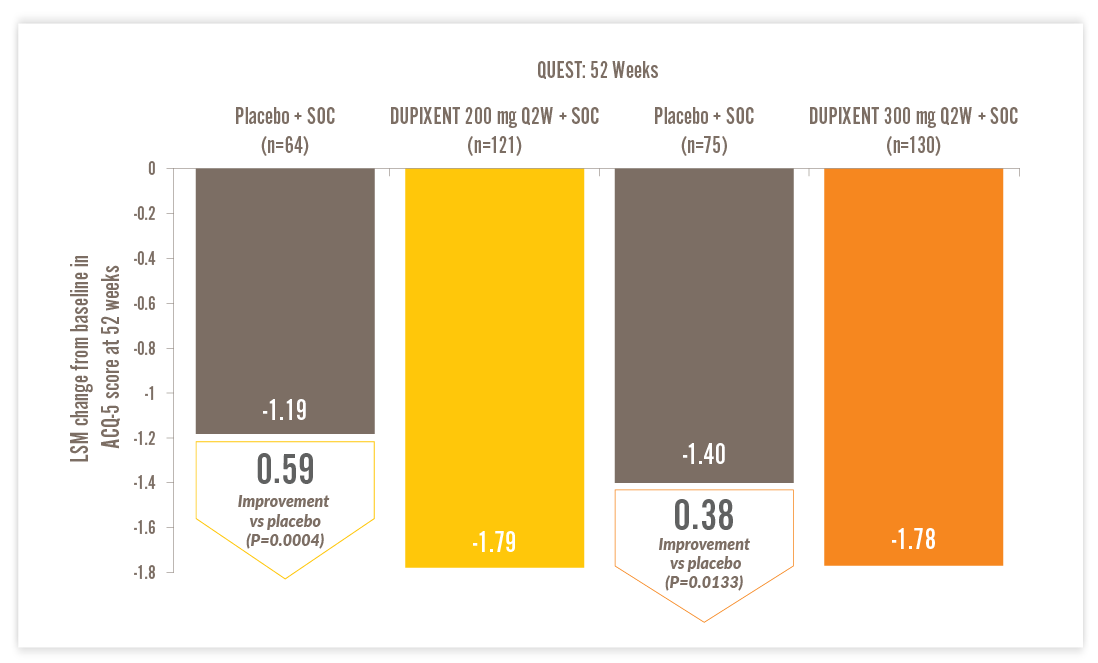
ACQ-5, Asthma Control Questionnaire-5; LSM, least squares mean; Q2W, every two weeks; SOC, standard of care
- ACQ-5 is a patient-reported measure of the adequacy of asthma control and change in asthma control that occurs either spontaneously or as a result of treatment. Higher scores indicate less control; a global score is calculated ranging from 0 to 6. The minimal clinically important difference is 0.514
- Results were derived from mixed-effects model with repeated measures with change from baseline in ACQ-5 score up to Week 52 as the response variable and treatment, age, region (pooled country), baseline eosinophil strata, visit, treatment-by-visit interaction, baseline ACQ-5 score and baseline-by-visit interaction as covariates14
DUPIXENT SIGNIFICANTLY REDUCED ASTHMA EXACERBATIONS VS PLACEBO1
IN A POST HOC ANALYSIS OF PATIENTS WITH EOS ≥150 CELLS/µL AND FeNO ≥25 PPB, AND HIGH-DOSE ICS AT BASELINE14
- Up to 1.16 (74% relative) reduction in annualised severe exacerbations with DUPIXENT 200 mg every two weeks (Q2W) + SOC vs placebo + SOC (0.41 vs 1.57) (P<0.0001)*14
Annualised rate of severe asthma exacerbations at Week 52†14
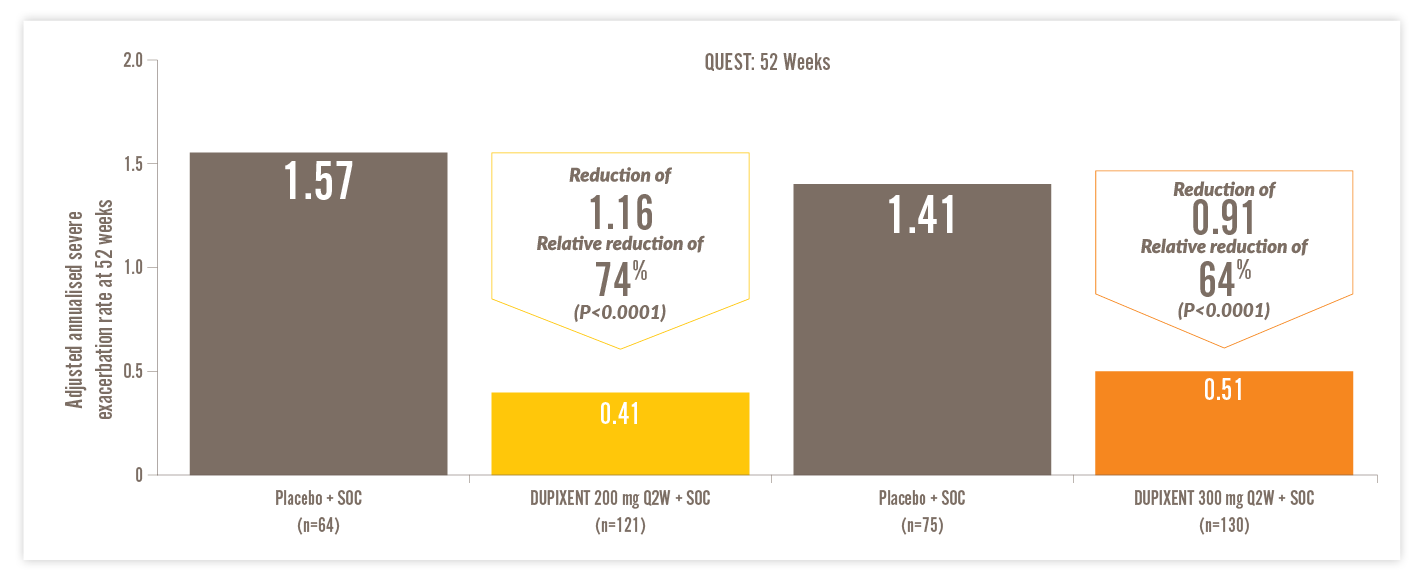
Q2W, every two weeks; SOC, standard of care
- A severe asthma exacerbation was defined as a deterioration of asthma leading to treatment for 3 days or more with systemic glucocorticoids or hospitalisation or an emergency department visit leading to treatment with systemic glucocorticoids14
- Annualised event rate was the total number of exacerbations that occurred during the treatment period divided by the total number of participant-years treated14
*SOC involved treatment with a high-dose inhaled glucocorticoid (fluticasone proportionate at a total daily dose of ≥1000 μg or equipotent equivalent) plus up to two additional controllers (e.g. LABA or LTRA).14 †74% reduction in annualised severe exacerbations at Week 52 (QUEST) with DUPIXENT 200 mg + SOC (n=121) vs placebo + SOC (n=64) (0.41 vs 1.57) (P<0.001), and 64% reduction with DUPIXENT 300 mg + SOC (n=130) vs placebo + SOC (n=75) (0.51 vs 1.41 (P<0.0001) in patients on high-dose ICS with EOS ≥150 cells/μL and FeNO ≥25 ppb (post hoc analysis).14
A severe asthma exacerbation was defined as a deterioration of asthma leading to treatment for 3 days or more with systemic glucocorticoids or hospitalisation or an emergency department visit leading to treatment with systemic glucocorticoids.14
The population in DUPIXENT asthma trials included patients on medium-to-high dose ICS. All data presented above represent the high-dose ICS population.14
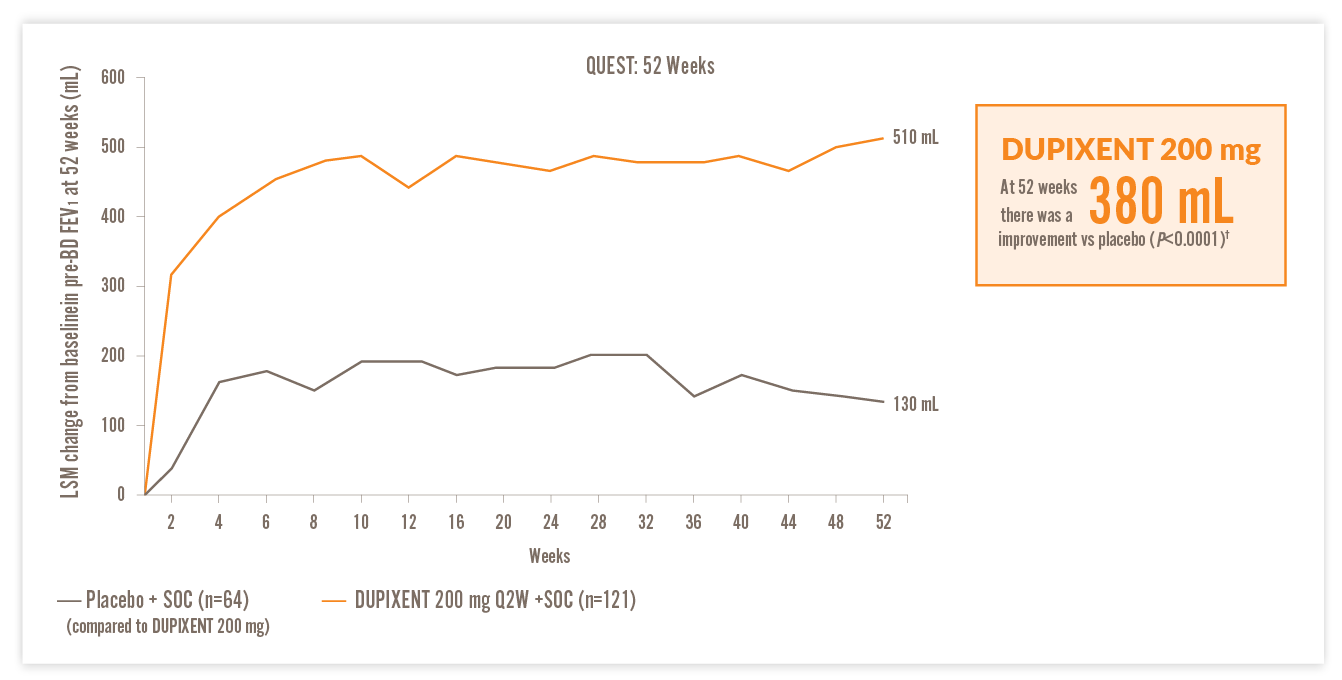
RAPID AND SUSTAINED IMPROVEMENT IN LUNG FUNCTION VS PLACEBO14
IN A POST HOC ANALYSIS OF PATIENTS WITH EOS ≥150 CELLS/µL AND FeNO ≥25 PPB, AND HIGH-DOSE ICS AT BASELINE14
200 mg DUPIXENT
300 mg DUPIXENT
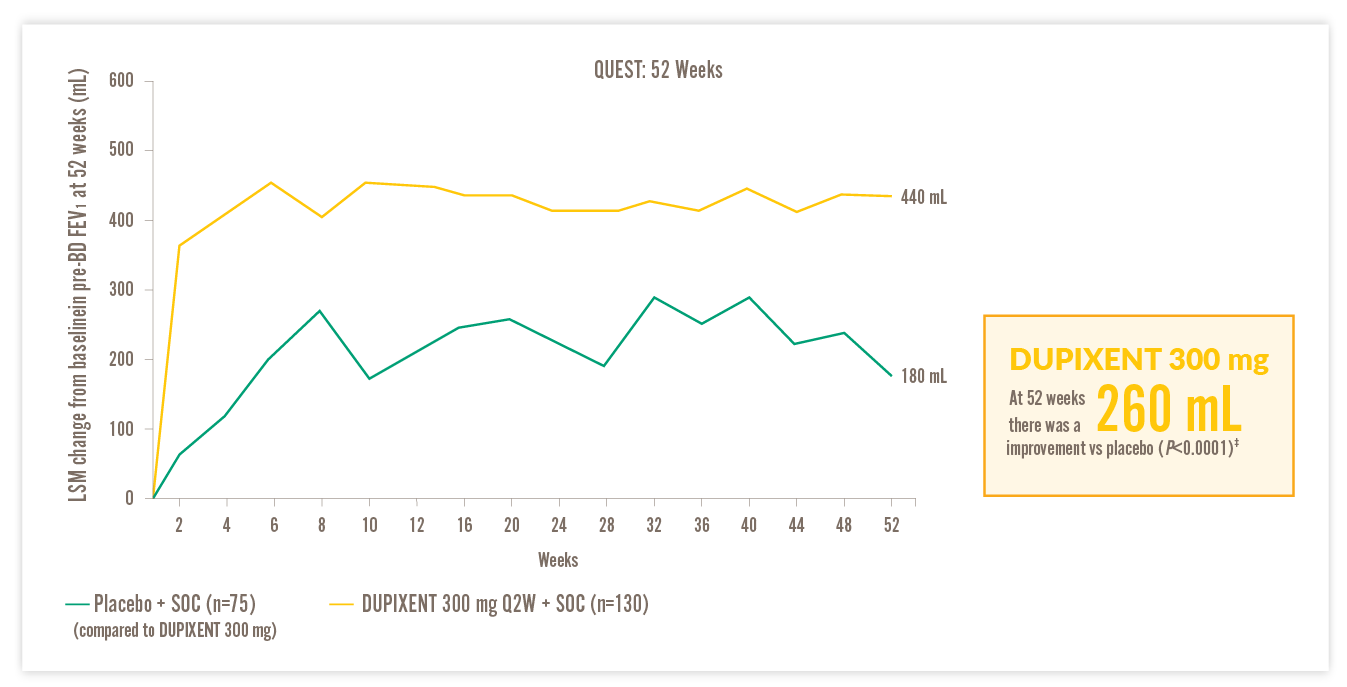
FEV1, forced expiratory volume in 1 second; LSM, least squares mean; Q2W, every two weeks; SOC, standard of care
†510 mL improvement from baseline in FEV1 at Week 52 with DUPIXENT 200 mg Q2W + SOC (n=93) vs 130 mL with placebo + SOC (n=48) (LSM difference, 380 mL [95% CI: 240, 520 mL]) in patients on high-dose ICS with EOS ≥150 cells/μL and FeNO ≥25 ppb.14 ‡440 mL improvement from baseline in FEV1 at Week 52 with DUPIXENT 300 mg Q2W + SOC (n=105) vs 180 mL with placebo + SOC (n=61) (LSM difference, 260 mL [95% CI: 130, 390 mL]) in patients on high-dose ICS with EOS ≥150 cells/μL and FeNO ≥25 ppb.14
Freya, 9
Child with Severe Asthma (6-11)

Diagnosed with severe asthma and moderate eczema
Currently on medium dose of ICS, LABA, and LTRA
Has considerable time off school due to her asthma and has to limit her participation in physical activities
Medical history
| Blood EOS levels | 200 cells/μL |
| Total lgE | 550 IU/mL |
| FeNO | 65 ppb |
| Severe exacerbations in the last year | 5 |
| Lung function | 60% FEV1 |
It is estimated that around 5-10% of children with asthma have disease characterised as severe15
Severe asthma in children is assocaited with:



VOYAGE TRIAL
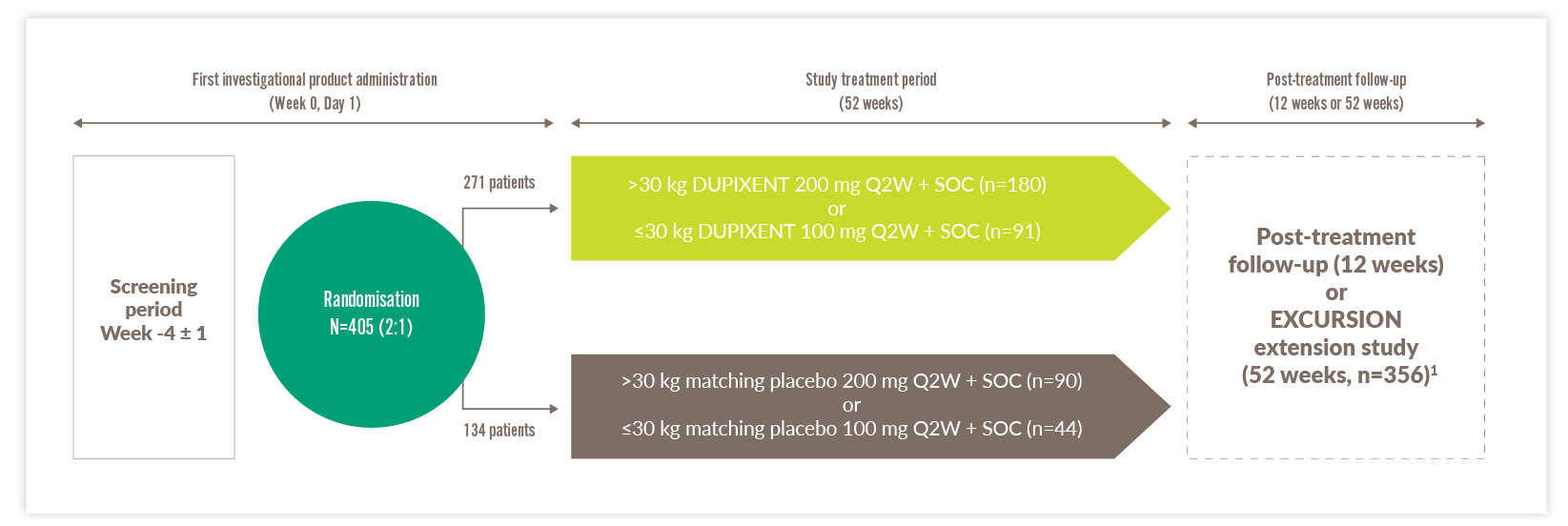
VOYAGE study design15,19
- The VOYAGE study was a randomised, double-blind, placebo-controlled, Phase 3 study designed to demonstrate the efficacy and safety of DUPIXENT over a period of up to 52 weeks in children 6–11 years of age with uncontrolled moderate-to-severe* asthma15
- Clinical trials included patients with uncontrolled moderate-to-severe asthma – study was designed prior to marketing authorisation. DUPIXENT is indicated in children 6–11 years old as add-on maintenance treatment for severe asthma with Type 2 inflammation characterised by raised blood EOS and/or raised FeNO, who are inadequately controlled with medium to high dose ICS plus another medicinal product for maintenance treatment*1
Q2W, every two weeks; SOC, standard of care
*Some patients in this study classed as having severe asthma no longer fall into this category, owing to changes in the definition of high-dose ICS.
- Primary analyses were based on 259 patients with baseline EOS ≥300 cells/μL and 350 patients with markers of Type 2 inflammation (baseline EOS ≥150 cells/μL or FeNO ≥20 ppb). There was no minimum biomarker requirement for enrolment. During the 52-week treatment period, patients received subcutaneous injections of DUPIXENT 100 mg or 200 mg Q2W, based on weight tier (100 mg for ≤30 kg, 200 mg for >30 kg), or placebo Q2W. The primary endpoint of the study was the annualised rate of severe asthma exacerbation events15,19
In the VOYAGE study, dupilumab pharmacokinetics was investigated in 270 patients with moderate-to-severe asthma following subcutaneous administration of either 100 mg Q2W (for 91 children weighing < 30 kg) or 200 mg Q2W (for 179 children weighing ≥ 30 kg). Simulation of a 300 mg Q4W subcutaneous dose in children aged 6 to 11 years with body weight of ≥ 15 kg to < 30 kg and ≥ 30 kg to < 60 kg resulted in predicted steady-state trough concentrations similar to the observed trough concentrations of 200 mg Q2W (≥ 30 kg) and 100 mg Q2W (< 30 kg), respectively. In addition, simulation of a 300 mg Q4W subcutaneous dose in children aged 6 to 11 years with body weight of ≥ 15 kg to < 60 kg resulted in predicted steady-state trough concentrations similar to those demonstrated to be efficacious in adults and adolescents. After the last steady state dose, the median time for dupilumab concentrations to decrease below the lower limit of detection, estimated by population PK analysis, was 14 to 18 weeks for 100 mg Q2W, 200 mg Q2W or 300 mg Q4W. 1
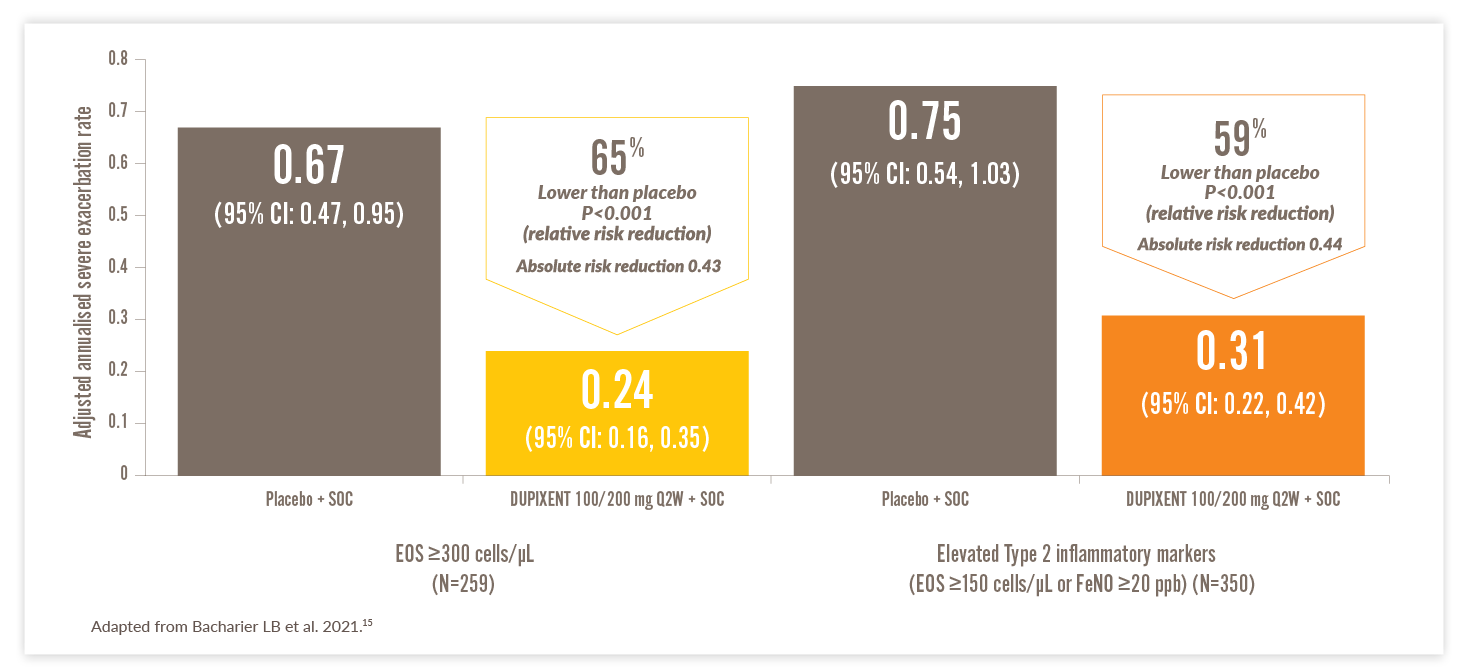
DUPIXENT SIGNIFICANTLY REDUCED ASTHMA EXACERBATIONS VS PLACEBO IN CHILDREN15
Significant annualised severe exacerbation reduction vs placebo through Week 52 (VOYAGE primary endpoint)*15
CI, confidence interval; EOS, eosinophils; FeNO, fraction of exhaled nitric oxide; Q2W, every two weeks; SOC, standard of care
Additional exacerbation-related endpoints
- In patients with EOS ≥150 cells/μL or FeNO ≥20 ppb, 77.1% (n=236) of children treated with DUPIXENT had no severe exacerbations over 52 weeks vs 59.6% (n=114) in the placebo group15
- In patients with EOS ≥300 cells/μL, the percentage was 79.0% and 58.3% in the two groups, respectively15
*As observed across a 52-week trial (VOYAGE) of paediatric patients.15
A severe asthma exacerbation was defined as a deterioration of asthma control leading to treatment with systemic glucocorticoids for at least 3 days, hospitalisation, or an emergency department visit leading to the systemic administration of glucocorticoids.15
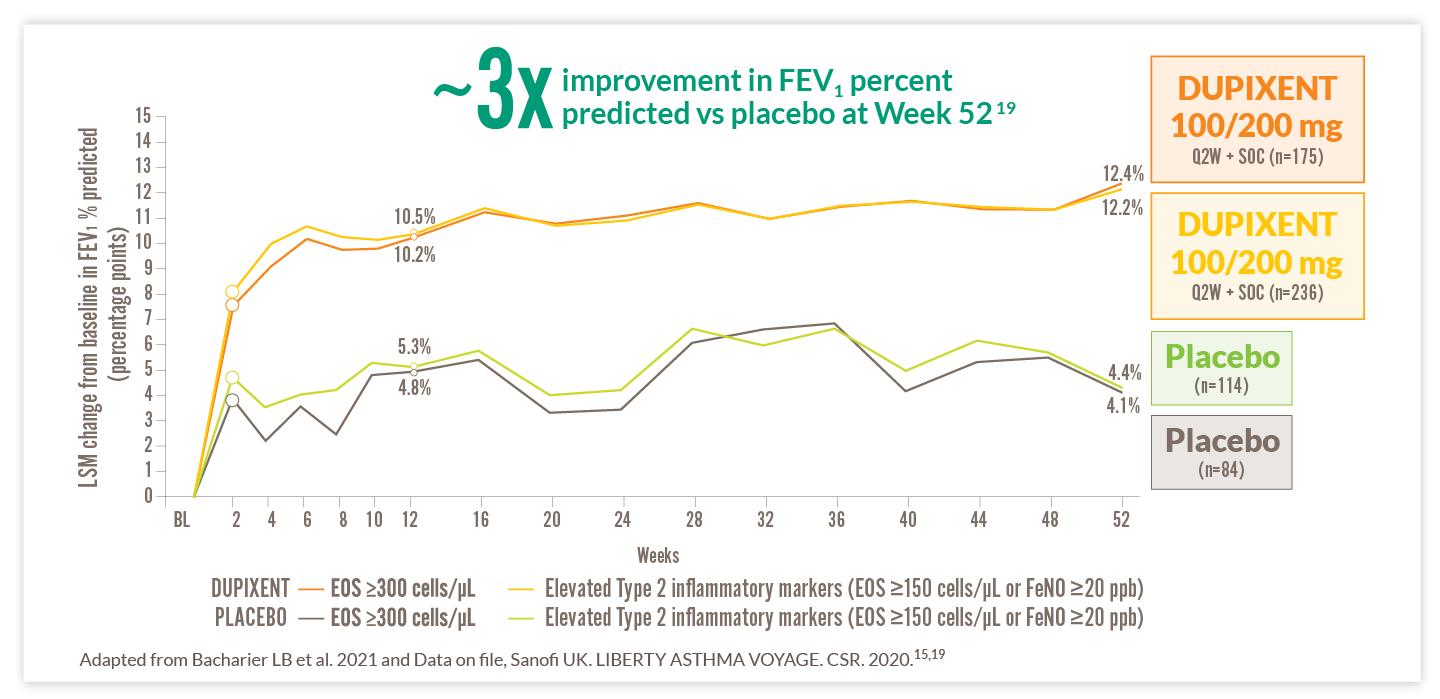
DUPIXENT IMPROVED LUNG FUNCTION VS PLACEBO IN CHILDREN15
PAEDIATRIC PATIENTS (6–11 YEARS) WITH ELEVATED BASELINE EOS SHOWED IMPROVEMENT VS PLACEBO AS EARLY AS WEEK 2, WITH SUSTAINED IMPROVEMENT THROUGH WEEK 52 (VOYAGE SECONDARY ENDPOINT)1,15
Sustained improvement in lung function over 52-week period (VOYAGE secondary endpoint)*15,19
EOS, eosinophils; FeNO, fraction of exhaled nitric oxide; FEV1, forced expiratory volume in 1 second; LSM, least squares mean; Q2W, every two weeks; SOC, standard of care
IN PATIENTS WITH THE TYPE 2 INFLAMMATORY PHENOTYPE, THOSE TREATED WITH DUPIXENT 100/200 MG REPORTED IMPROVED LUNG FUNCTION MEASURED BY FEV1 PERCENT PREDICTED VS PATIENTS TREATED WITH PLACEBO AS EARLY AS WEEK 2 AND EFFECTS WERE MAINTAINED THROUGHOUT THE 52-WEEK TREATMENT DURATION (8.1% VS 4.8%, RESPECTIVELY)†15
*FEV1 percent predicted is a common endpoint in paediatric asthma trials to evaluate change in lung function vs predicted lung function based on several factors – including age, height, and sex – to account for children’s growing lung capacity at different stages of development.19 †DUPIXENT n=236 vs placebo n=114.19
DUPIXENT REDUCED THE DAILY BURDEN OF SEVERE ASTHMA FOR PAEDIATRIC PATIENTS AND THEIR CAREGIVERS VS PLACEBO1
IN PATIENTS WITH EOS ≥150 CELLS/µL OR FeNO ≥20 PPB
Combined results for DUPIXENT 100/200mg*,**
of patients improved by at least 0.5 points in ACQ-7-IA (seven questions about symptoms, lung function and medication use) vs 69% with placebo*1
of patients improved by at least 0.5 points in PAQLQ(S)-IA (23 questions in three domains: symptoms, activity limitations and emotional function) vs 65% with placebo*1
Improvement in PACQLQ (13 questions in two domains: activity limitation and emotional function) in caregivers vs placebo*1
Primary endpoint was met.
*79% of patients with EOS ≥150 cells/μL or FeNO ≥20 ppb improved with DUPIXENT 100/200 mg + SOC (n=236) vs 69% with placebo + SOC (n=114), odds ratio vs placebo 1.82 (95% CI: 1.02, 3.24).1 †73% of patients with EOS ≥150 cells/μL or FeNO ≥20 ppb improved with DUPIXENT 100/200 mg + SOC (n=211) vs 65% with placebo + SOC (n=107), odds ratio vs placebo 1.57 (95% CI: 0.87, 2.84).1 ‡Paediatric patients with EOS ≥150 cells/μL or FeNO ≥20 ppb had 0.47 LSM difference vs placebo (95% CI: 0.22, 0.72).1
**In the VOYAGE study, dupilumab pharmacokinetics was investigated in 270 patients with moderate-to-severe asthma following subcutaneous administration of either 100 mg Q2W (for 91 children weighing < 30 kg) or 200 mg Q2W (for 179 children weighing ≥ 30 kg). Simulation of a 300 mg Q4W subcutaneous dose in children aged 6 to 11 years with body weight of ≥ 15 kg to < 30 kg and ≥ 30 kg to < 60 kg resulted in predicted steady-state trough concentrations similar to the observed trough concentrations of 200 mg Q2W (≥ 30 kg) and 100 mg Q2W (< 30 kg), respectively. In addition, simulation of a 300 mg Q4W subcutaneous dose in children aged 6 to 11 years with body weight of ≥ 15 kg to < 60 kg resulted in predicted steady-state trough concentrations similar to those demonstrated to be efficacious in adults and adolescents. After the last steady state dose, the median time for dupilumab concentrations to decrease below the lower limit of detection, estimated by population PK analysis, was 14 to 18 weeks for 100 mg Q2W, 200 mg Q2W or 300 mg Q4W. 1
Jane, 35
OCS-dependent Patient with Severe Asthma (12+) SCOTLAND ONLY

Diagnosed with severe asthma
Previous biologic treatment with omalizumab
On long-term, continued OCS treatment in the form of prednisolone
Social life is negatively impacted by side effects of her maintenance OCS treatment
Medical history
| Blood EOS levels | 150 cells/μL |
| FeNO | 25 ppb |
| Severe exacerbations in the last year | ≥4 exacerbations in the 12 months prior to starting first biologic |
86% of patients reduced or eliminated OCS use by Week 24 with DUPIXENT 300 mg + SOC vs 68% with placebo + SOC (P<0.001).20
For patients with severe asthma (12+) and who are on oral corticosteroids, an initial dose of 600 mg (two 300 mg injections), followed by 300 mg every other week administered as subcutaneous injection.
PER GINA, MAINTENANCE OCS SHOULD BE CONSIDERED ONLY AS A LAST RESORT.7
93% of patients with severe asthma have ≥1 side effects linked to OCS exposure.21
Long-term side effects of OCS can include:7,21,22
| MENTAL | PHYSICAL |
|
|
Even the short-term use of OCS can lead to:7,23
| MENTAL | PHYSICAL |
|
|
VENTURE TRIAL
VENTURE was a 24-week,* randomised, double-blind, placebo-controlled, Phase 3 study in patients (12+) with severe asthma and patients were enrolled irrespective of baseline blood EOS counts or other Type 2 biomarkers20
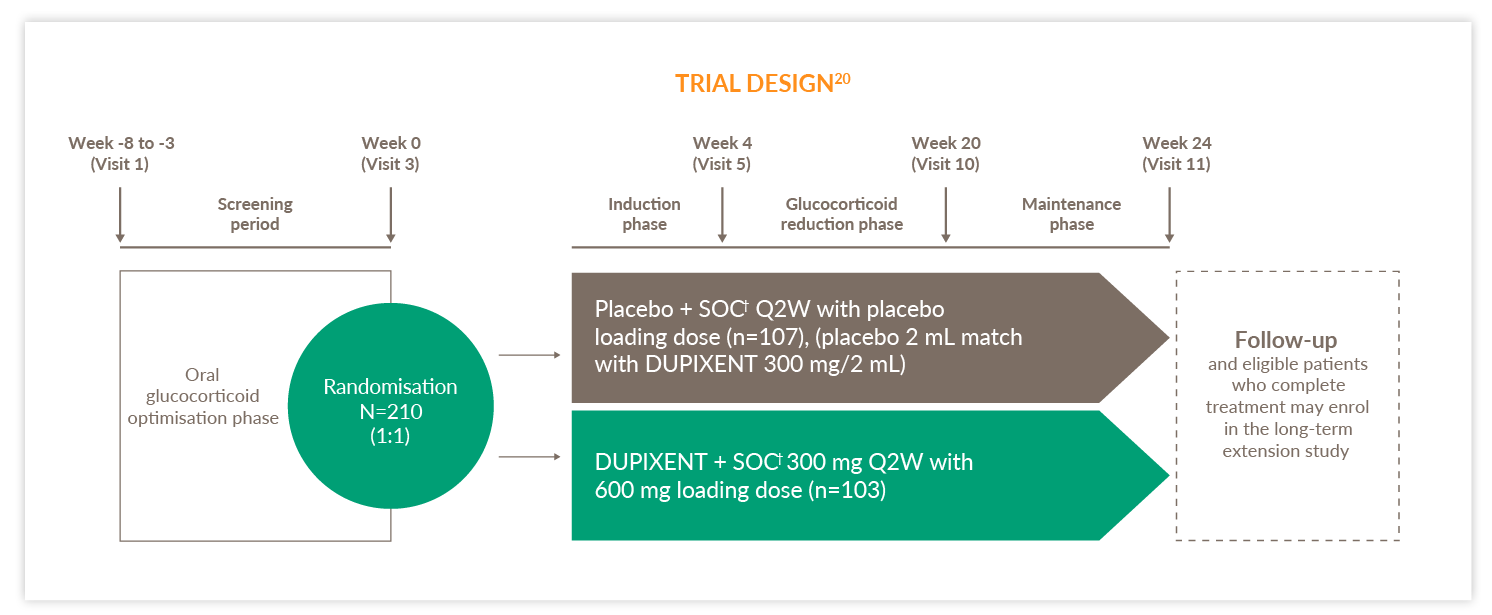
Q2W, every two weeks; SOC, standard of care
Primary endpoint20
- Percentage reduction of OCS dose from baseline at Week 24 while maintaining asthma control (was met)
*VENTURE consisted of a 24-week treatment phase, followed by a 12-week evaluation phase.20 †SOC involved treatment with a high-dose inhaled glucocorticoid (fluticasone propionate at a total daily dose of >500 μg or equipotent equivalent) in combination with up to 2 controllers (i.e. a LABA or LTRA) and daily oral glucocorticoids (i.e. 5–35 mg per day of prednisone or prednisolone or equivalent).20
TRAVERSE TRIAL
Extension trial of patients from DRI12544, QUEST, and VENTURE1,20,24,25
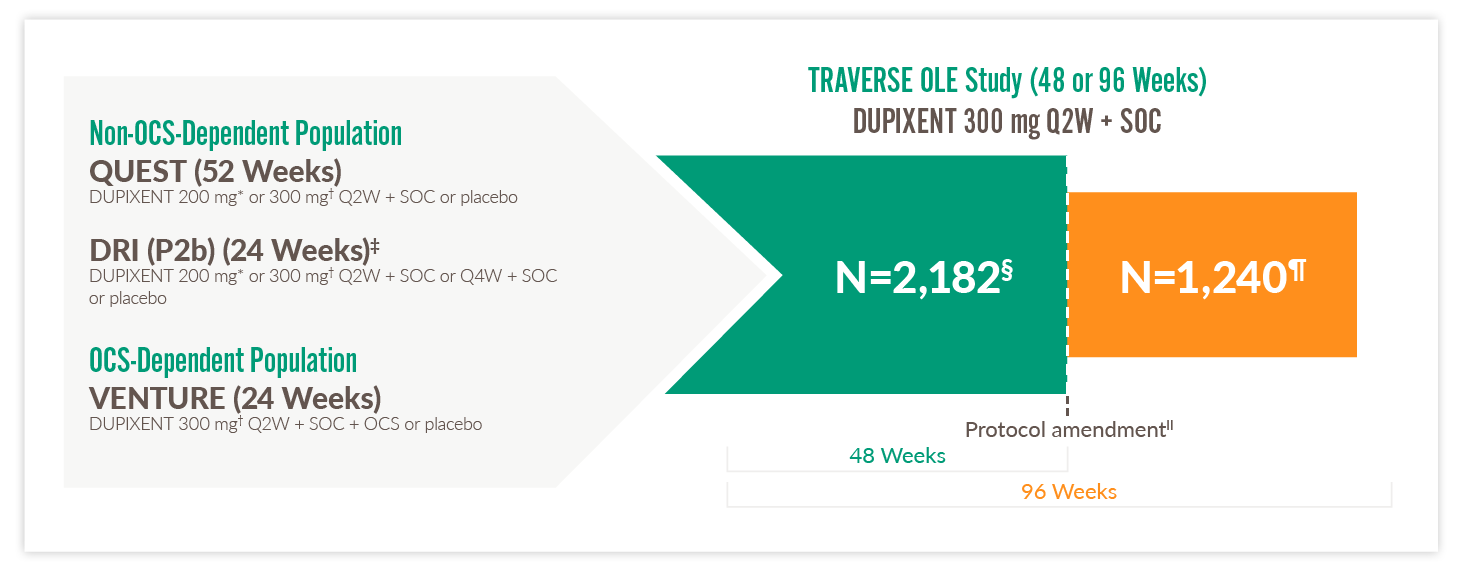
OCS, oral corticosteroids; OLE, open-label extension; Q2W, every two weeks; Q4W, every four weeks; SOC, standard of care
Primary endpoint25
- The number and percentage of patients with any treatment-emergent adverse events
Select other endpoints25
- Number and annualised rate of severe exacerbation events
- Improvement in FEV1
- TRAVERSE evaluated the long-term safety, tolerability, and efficacy of DUPIXENT in adults and adolescents who enrolled from a previous DUPIXENT study, including DRI12544, QUEST, and VENTURE24,25
- In the OCS-dependent population, percent reduction from baseline in OCS dose and proportions of patients achieving ≥50% reduction and completely tapering off OCS24
*With 400 mg loading dose.1 †With 600 mg loading dose.1 ‡It should be noted that eligible patients completed the 16-week, post-treatment, follow-up period of the parent study before being rolled over into TRAVERSE.25 §Total number of subjects enrolled and exposed to treatment in OLE.25 ¶Total number of subjects who continued to be exposed to treatment beyond 48 weeks.25 ||The treatment period was shortened to 48 weeks after a protocol amendment. Patients enrolled in the TRAVERSE study before the amendment were treated for 96 weeks, whereas those enrolled after the amendment were treated for 48 weeks.25
IN OCS-DEPENDENT SEVERE ASTHMA PATIENTS, DUPIXENT IS A GINA-RECOMMENDED BIOLOGIC7
By Week 24 in the LIBERTY ASTHMA VENTURE (VENTURE) study:
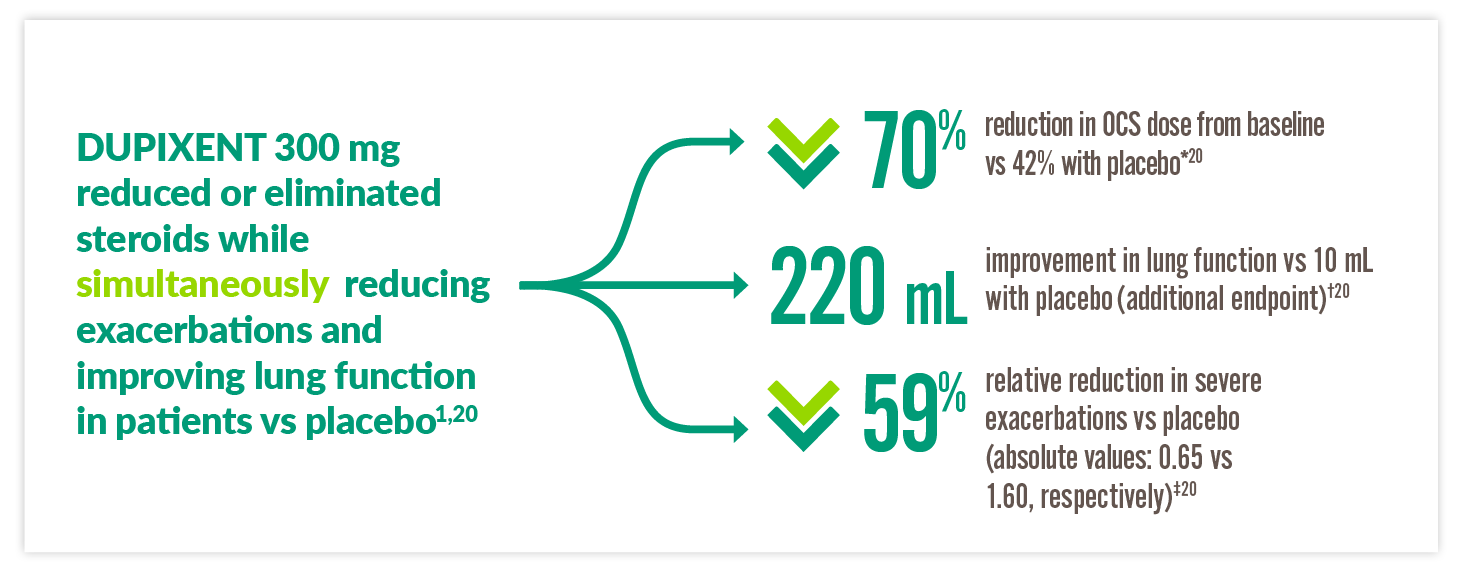
*70% reduction in OCS use from baseline by Week 24 with DUPIXENT 300 mg + standard of care (SOC) (n=103) vs 42% with placebo + SOC (n=107) (P<0.001).20 †220 mL improvement in pre-bronchodilator FEV1 at Week 24 with DUPIXENT 300 mg + SOC (n=103) vs 10 mL with placebo + SOC (n=107) (LSM difference, 220 mL [95% CI: 90, 340 mL]).20 ‡59% reduction in annualised rate of severe exacerbations at Week 24 with DUPIXENT 300 mg + SOC (n=103) vs placebo + SOC (n=107) (0.65 vs 1.60; rate ratio: 0.41 [95% CI: 0.26, 0.63]).20
DUPIXENT MAINTAINED SEVERE ASTHMA CONTROL OVER 2 YEARS24
By Week 96 in a subgroup analysis of VENTURE patients who continued to the LIBERTY ASTHMA TRAVERSE (TRAVERSE) study:24
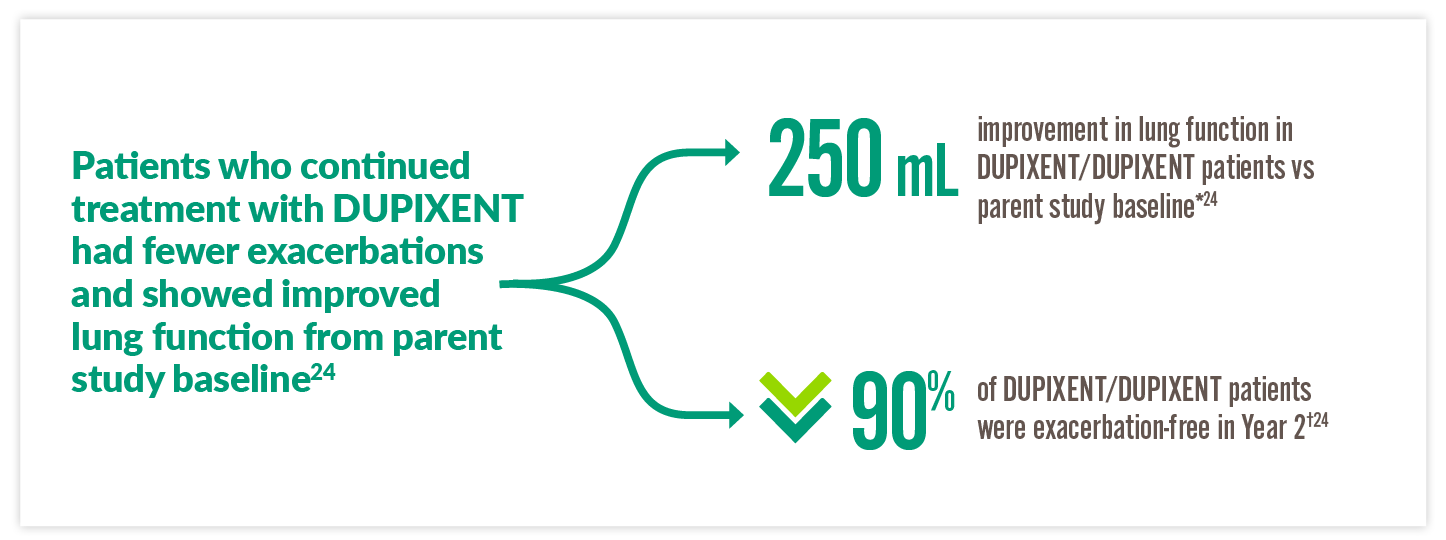
**250 mL improvement in FEV1 at Week 96 of TRAVERSE from VENTURE baseline in the DUPIXENT/DUPIXENT patient group (n=28) vs 360 mL in the placebo/DUPIXENT patient group (n=32).24 †Of the 67 DUPIXENT/DUPIXENT patients who were treated up to 96 weeks in TRAVERSE, 90% were exacerbation-free during Weeks 48 through 96 of TRAVERSE.24
Legacy
Over 1,000,000 PATIENTS AND COUNTING5,a





a 1,017,901 patients treated worldwide across all indications (as of August 2024).5
b In patients aged 6 months to 11 years. DUPIXENT is indicated for moderate-to-severe atopic dermatitis in patients 12+ years.1
c OCS-dependent as specified in GINA guidelines.7
d As observed across 3 randomised, placebo-controlled, multicentre trials of 24 to 52 week's duration (DR11 2544, QUEST, and VENTURE) and a 96-week, open-label extension trial (TRAVERSE) of adult and adolescent patients. The safety profile observed in the paediatric asthma clinical study (a 52-week multicentre, randomised, double-blind, placebo-controlled study [VOYAGE]) was similar to that seen in adults.1
e In adults with moderate-to-severe atopic dermatitis, the safety and tolerability profile of DUPIXENT has been studied for 4 years in an open-label extension study and was generally consistent with the 52-week data.1
INDICATION STATEMENTS
Adults and adolescents
Dupixent is indicated for the treatment of moderate-to-severe atopic dermatitis in adults and adolescents 12 years and older who are candidates for systemic therapy.
Children 6 months to 11 years of age
Dupixent is indicated for the treatment of severe atopic dermatitis in children 6 months to 11 years old who are candidates for systemic therapy.
Adults and adolescents
Dupixent is indicated in adults and adolescents 12 years and older as add-on maintenance treatment for severe asthma with type 2 inflammation characterised by raised blood eosinophils and/or raised who are inadequately controlled with high dose plus another medicinal product for maintenance treatment.
Children 6 to 11 years of age
Dupixent is indicated in children 6 to 11 years old as add-on maintenance treatment for severe asthma with type 2 inflammation characterised by raised blood eosinophils and/or raised fraction of exhaled nitric oxide (FeNO), who are inadequately controlled with medium to high-dose ICS plus another medicinal product for maintenance treatment.
Dupixent is indicated as an add-on therapy with intranasal corticosteroids for the treatment of adults with severe CRSwNP for whom therapy with systemic corticosteroids and/or surgery do not provide adequate disease control.
Dupixent does not have reimbursement for CRSwNP in the United Kingdom.
Dupixent is indicated for the treatment of adults with moderate-to-severe prurigo nodularis who are candidates for systemic therapy.
Dupixent is accepted for use within NHS Scotland. For the full guidance please follow this link for guidance for SMC 2598.27
Dupixent is not recommended, within its marketing authorisation in England, for treating moderate to severe prurigo nodularis in adults when systemic treatment is suitable. For full guidance please follow this link for guidance for TA - 955.28
Dupixent is indicated for the treatment of eosinophilic esophagitis in adults and adolescents 12 years and older, weighing at least 40 kg, who are inadequately controlled by, are intolerant to, or who are not candidates for conventional medicinal therapy.
Dupixent does not have reimbursement for eosinophilic esophagitis in the United Kingdom.
Safety
DUPIXENT SAFETY PROFILE IN SEVERE ASTHMA HAS BEEN RESEARCHED FOR OVER 3 YEARS.25,a
DUPIXENT was generally well tolerated in adult and adolescent patients with a similar safety profile in the paediatric asthma population.1
System Organ Class |
Frequencyb |
Adverse Reactions |
| Infections and infestations | Common |
Conjunctivitis c Oral herpes c |
| Blood and lymphatic system disorders | Common | Eosinophilia |
| Immune system disorders |
Uncommon
|
Angioedema d Anaphylactic reaction |
| Eye disorders |
Common
| Conjunctivitis allergic c Keratitis c, d Blepharitis c,e Eye pruritus c, e Dry eye c, e Ulcerative keratitis c,d,e |
| Skin and subcutaneous tissue disorders | Uncommon | Facial rash d |
| Musculoskeletal and connective tissue disorders | Common | Arthralgia d |
| General disorders and administration site conditions | Common | Injection site reactions (includes erythema, oedema, pruritus, pain, swelling, and bruising) |
a As observed across 3 randomised, placebo-controlled, multi-centre trials of 24 to 52 weeks’ duration (DRI12544, QUEST, and VENTURE), and a 96-week, open-label extension trial (TRAVERSE) of adult and adolescent patients. The safety profile observed in the paediatric asthma clinical study (a 52-week multicentre, randomised, double blind, placebo-controlled study [VOYAGE]) was similar to that seen in adults.
bCommon (≥1/100 to <1/10); Uncommon (≥1/1,000 to <1/100); Rare (≥1/10,000 to <1/1,000).
ceye disorders and oral herpes occurred predominately in atopic dermatitis studies.
dfrom postmarketing reporting.
ethe frequencies for eye pruritus, blepharitis, and dry eye were common and ulcerative keratitis was uncommon in atopic dermatitis studies.
Adverse reactions for DUPIXENT in children aged 6 to 11 years1
- Similar results were observed in children 6 to 11 years of age with moderate-to-severe asthma as in adults and adolescents 12 years and older
- The additional adverse reaction of enterobiasis was reported in 1.8% (5 patients) in the DUPIXENT groups and none in the placebo group
- All enterobiasis cases were mild-to-moderate and patients recovered with anti-helminth treatment without DUPIXENT treatment discontinuation
Special Warnings1
- DUPIXENT should not be used to treat acute asthma symptoms or acute exacerbations
- DUPIXENT should not be used to treat acute bronchospasm or status asthmaticus
- If a systemic hypersensitivity reaction (immediate or delayed) occurs, DUPIXENT should be discontinued immediately, and appropriate therapy initiated
- Systemic, topical, or inhaled corticosteroids (ICS) should not be discontinued abruptly upon initiation of therapy with DUPIXENT
- Reductions in corticosteroid dose, if appropriate, should be gradual and performed under the direct supervision of a physician
- Reduction in corticosteroid dose may be associated with systemic withdrawal symptoms and/or unmask conditions previously suppressed by systemic corticosteroid therapy
- If a systemic hypersensitivity reaction (immediate or delayed) occurs, DUPIXENT should be discontinued immediately, and appropriate therapy initiated
- Biomarkers of Type 2 inflammation may be suppressed by systemic corticosteroid use; this should be taken into consideration to determine the Type 2 status in patients taking oral corticosteroids (OCS)
- Patients who develop conjunctivitis that does not resolve following standard treatment or signs and symptoms suggestive of keratitis should undergo ophthalmological examination, as appropriate
- Patients with pre-existing helminth infections should be treated before initiating DUPIXENT. If patients become infected while receiving treatment with DUPIXENT and do not respond to anti-helminth treatment, DUPIXENT should be discontinued until infection resolves
- Concurrent use of live and live attenuated vaccines with dupilumab should be avoided as clinical safety and efficacy have not been established. It is recommended that patients should be brought up to date with live and live attenuated immunisations in agreement with current immunisation guidelines prior to treatment with DUPIXENT
- In order to improve the traceability of biological medicinal products, the name and the batch number of the administered product should be clearly recorded
DUPIXENT is not an immunosuppressant.1
Dosing
YOU DECIDE: PATIENTS OR CAREGIVERS CAN ADMINISTER AT HOME OR YOU CAN ADMINISTER IN YOUR PRACTICE.1
*200 mg=1.14 mL solution.1
†Advise AD patients with co-morbid asthma not to adjust or stop their asthma treatments without consultation with their physicians. For patients with severe asthma and who are on OCS, or for patients with severe asthma and co-morbid moderate-to-severe AD or adults with co-morbid severe CRSwNP, use an initial dose of 600 mg (two 300 mg injections), followed by 300 mg every other week, which should be administered as a subcutaneous injection.1
‡300 mg=2 mL solution.1
AD, atopic dermatitis; CRSwNP, chronic rhinosinusitis with nasal polyposis.
*300 mg=2 mL solution.1
†200 mg=1.14 mL solution.1
DUPIXENT Pre-filled Pen1,29,a
- Single-press auto-injector
- A clear 2-step process
- Visual and audible feedback
- Compact and convenient to carry
- Hidden needle
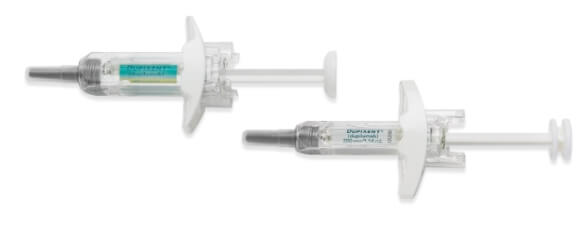
DUPIXENT Pre-filled Syringe1,30,b
- Manual control of injection speed
- Finger grip for comfort
- Visual confirmation of injection delivery
- Needle shield
- Easy-to-carry format
aThe pre-filled pen is only available for patients 2 years and older.
bThe pre-filled syringe is available for patients 6 months and older.
*200 mg=1.14 mL solution.1
†Advise AD patients with co-morbid asthma not to adjust or stop their asthma treatments without consultation with their physicians. For patients with severe asthma and who are on OCS, or for patients with severe asthma and co-morbid moderate-to-severe AD or adults with co-morbid severe CRSwNP, use an initial dose of 600 mg (two 300 mg injections), followed by 300 mg every other week, which should be administered as a subcutaneous injection.1
‡300 mg=2 mL solution.1
AD, atopic dermatitis; CRSwNP, chronic rhinosinusitis with nasal polyposis.
*300 mg=2 mL solution.1
†200 mg=1.14 mL solution.1
DUPIXENT Pre-filled Pen1,29,a
- Single-press auto-injector
- A clear 2-step process
- Visual and audible feedback
- Compact and convenient to carry
- Hidden needle

DUPIXENT Pre-filled Syringe1,30,b
- Manual control of injection speed
- Finger grip for comfort
- Visual confirmation of injection delivery
- Needle shield
- Easy-to-carry format

aThe pre-filled pen is only available for patients 2 years and older.
bThe pre-filled syringe is available for patients 6 months and older.
DUPIXENT is intended for use under the guidance of a healthcare provider.1
- Physicians or nurses should provide proper training to patients and/or caregivers on the preparation and administration of DUPIXENT prior to use, according to the Instructions for Use.
- DUPIXENT can be administered in the practice under the guidance of a healthcare provider if the patient or caregiver is not an appropriate candidate to administer the injection.
For all administration instructions, please click here for the UK Summary of Product Characteristics.1
Reimbursement
DUPIXENT is recommended for specific use in England and Wales31
ADULTS AND ADOLESCENTS (12+)
NICE Technology Appraisal Guidance (TA751)31
DUPIXENT is recommended by National Institute for Health and Care Excellence (NICE), as an add-on maintenance therapy option for treating severe asthma with Type 2 inflammation that is inadequately controlled in people 12 years and over, despite maintenance therapy with high-dose inhaled corticosteroids and another maintenance treatment, only if the below criteria are met.31
- The person has agreed to and followed the optimised standard treatment plan.
- The dosage used is 400 mg initially and then 200 mg subcutaneously every other week.
- Blood eosinophils (EOS) ≥ 150 cells/μL and fraction of exhaled nitric oxide (FeNO) ≥ 25 ppb.
- The person has had ≥4 exacerbations in the preceding year.
- The person is not eligible for treatment with an anti-interleukin-5 therapy, or their asthma has not responded adequately to anti-interleukin-5 therapy.
- The company provides DUPIXENT with the discount agreed in the patient access scheme (PAS).
Stop dupilumab if the rate of severe asthma exacerbations has not been reduced by at least a 50% after 12 months.
CHILDREN (6 - 11 years old)
DUPIXENT is reimbursed through the Medicines for Children (M4C) policy.
DUPIXENT qualifies for M4C reimbursement as a medication approved in adults by a NICE technology assessment (TA) and one that has a license for use in children and both the indication for use and the age of the child fall within those specified in the adult license.32
M4C additional criteria32
- The patient meets all the NICE TA/NHS England clinical commissioning policy criteria for the proposed medicine/indication.
- The patient does not meet any exclusion criteria for the medicine indication in question.
- The use of the drug has been discussed at a multidisciplinary team (MDT) meeting which must include at least two consultants in the subspecialty with active and credible expertise in the relevant field of whom at least one must be a consultant paediatrician. The MDT should include a paediatric pharmacist and other professional groups appropriate to the disease area.
- The patient has been registered via the NHS England prior approval web-based system.
DUPIXENT has specific guidance for use in Scotland4
In Scotland, DUPIXENT is accepted for restricted use for severe asthma in patients 12 years and older with blood eosinophils (EOS) ≥150 cells/mL and fraction of exhaled nitric oxide (FeNO) ≥25 ppb, and ≥4 exacerbations in the preceding year, who have previously received biologic treatment with anti-IgE or anti-IL-5 therapies4
Scottish Medicines Consortium (SMC) advice4
- SMC reviewed DUPIXENT under the context of its indication: as add-on maintenance treatment for severe asthma in adults and adolescents 12 years and older with Type 2 inflammation characterised by raised blood EOS and/or raised FeNO, who are inadequately controlled with high dose inhaled corticosteroids (ICS) plus another medicinal product for maintenance treatment.
- The SMC advice applies to an approved Patient Access Scheme (PAS) that delivers the cost-effectiveness of DUPIXENT or a PAS/list price that is equivalent or lower
DUPIXENT is recommended for specific use in Northern Ireland33
ADULTS AND ADOLESCENTS (12+)
In Northern Ireland, DUPIXENT is accepted for use as add-on maintenance therapy as an option for treating severe asthma with Type 2 inflammation that is inadequately controlled in people 12 years and over, despite maintenance therapy with high-dose ICS and another maintenance treatment, only if the below criteria are met.33
Northern Ireland Formulary criteria:33
- The person has agreed to and follows an optimised standard treatment plan
- The dosage used is 400 mg initially and then 200 mg subcutaneously every other week
- The person has a blood EOS ≥150 cells/μL and FeNO ≥25 ppb, and has had ≥4 exacerbations in the preceding year
- The person is not eligible for biologics or has asthma that has not responded adequately to these biologic therapies
- The company provides DUPIXENT with the discount agreed in the PAS
For further information see NICE TA75116.
CHILDREN (6 - 11 years old)
In Northern Ireland, DUPIXENT is accepted for restricted use in children 6–11 years old as add-on maintenance treatment for severe asthma with Type 2 inflammation characterised by raised blood EOS and/or raised FeNO who are inadequately controlled with medium to high-dose ICS plus another medicinal product for maintenance treatment.33
Northern Ireland Formulary criteria:33
- This recommendation applies only in circumstances where the approved PAS is utilised or where the list/contract price is equivalent or lower than the PAS price
- Where infrastructure is in place and the service has capacity, interim commissioning of this drug is accepted on a cost-per-case basis
DUPIXENT is recommended for specific use in England and Wales31
ADULTS AND ADOLESCENTS (12+)
NICE Technology Appraisal Guidance (TA751)31
DUPIXENT is recommended by National Institute for Health and Care Excellence (NICE), as an add-on maintenance therapy option for treating severe asthma with Type 2 inflammation that is inadequately controlled in people 12 years and over, despite maintenance therapy with high-dose inhaled corticosteroids and another maintenance treatment, only if the below criteria are met.31
- The person has agreed to and followed the optimised standard treatment plan.
- The dosage used is 400 mg initially and then 200 mg subcutaneously every other week.
- Blood eosinophils (EOS) ≥ 150 cells/μL and fraction of exhaled nitric oxide (FeNO) ≥ 25 ppb.
- The person has had ≥4 exacerbations in the preceding year.
- The person is not eligible for treatment with an anti-interleukin-5 therapy, or their asthma has not responded adequately to anti-interleukin-5 therapy.
- The company provides DUPIXENT with the discount agreed in the patient access scheme (PAS).
Stop dupilumab if the rate of severe asthma exacerbations has not been reduced by at least a 50% after 12 months.
CHILDREN (6 - 11 years old)
DUPIXENT is reimbursed through the Medicines for Children (M4C) policy.
DUPIXENT qualifies for M4C reimbursement as a medication approved in adults by a NICE technology assessment (TA) and one that has a license for use in children and both the indication for use and the age of the child fall within those specified in the adult license.32
M4C additional criteria32
- The patient meets all the NICE TA/NHS England clinical commissioning policy criteria for the proposed medicine/indication.
- The patient does not meet any exclusion criteria for the medicine indication in question.
- The use of the drug has been discussed at a multidisciplinary team (MDT) meeting which must include at least two consultants in the subspecialty with active and credible expertise in the relevant field of whom at least one must be a consultant paediatrician. The MDT should include a paediatric pharmacist and other professional groups appropriate to the disease area.
- The patient has been registered via the NHS England prior approval web-based system.
DUPIXENT has specific guidance for use in Scotland4
In Scotland, DUPIXENT is accepted for restricted use for severe asthma in patients 12 years and older with blood eosinophils (EOS) ≥150 cells/mL and fraction of exhaled nitric oxide (FeNO) ≥25 ppb, and ≥4 exacerbations in the preceding year, who have previously received biologic treatment with anti-IgE or anti-IL-5 therapies4
Scottish Medicines Consortium (SMC) advice4
- SMC reviewed DUPIXENT under the context of its indication: as add-on maintenance treatment for severe asthma in adults and adolescents 12 years and older with Type 2 inflammation characterised by raised blood EOS and/or raised FeNO, who are inadequately controlled with high dose inhaled corticosteroids (ICS) plus another medicinal product for maintenance treatment.
- The SMC advice applies to an approved Patient Access Scheme (PAS) that delivers the cost-effectiveness of DUPIXENT or a PAS/list price that is equivalent or lower
DUPIXENT is recommended for specific use in Northern Ireland33
ADULTS AND ADOLESCENTS (12+)
In Northern Ireland, DUPIXENT is accepted for use as add-on maintenance therapy as an option for treating severe asthma with Type 2 inflammation that is inadequately controlled in people 12 years and over, despite maintenance therapy with high-dose ICS and another maintenance treatment, only if the below criteria are met.33
Northern Ireland Formulary criteria:33
- The person has agreed to and follows an optimised standard treatment plan
- The dosage used is 400 mg initially and then 200 mg subcutaneously every other week
- The person has a blood EOS ≥150 cells/μL and FeNO ≥25 ppb, and has had ≥4 exacerbations in the preceding year
- The person is not eligible for biologics or has asthma that has not responded adequately to these biologic therapies
- The company provides DUPIXENT with the discount agreed in the PAS
For further information see NICE TA75116.
CHILDREN (6 - 11 years old)
In Northern Ireland, DUPIXENT is accepted for restricted use in children 6–11 years old as add-on maintenance treatment for severe asthma with Type 2 inflammation characterised by raised blood EOS and/or raised FeNO who are inadequately controlled with medium to high-dose ICS plus another medicinal product for maintenance treatment.33
Northern Ireland Formulary criteria:33
- This recommendation applies only in circumstances where the approved PAS is utilised or where the list/contract price is equivalent or lower than the PAS price
- Where infrastructure is in place and the service has capacity, interim commissioning of this drug is accepted on a cost-per-case basis
ACQ-5, Asthma Control Questionnaire-5;
ACQ-7-IA, Asthma Control Questionnaire 7-Interviewer Administered;
AD, atopic dermatitis;
AERD, aspirin-exacerbated respiratory disease;
BD, bronchodilator;
BTS, British Thoracic Society;
CI, confidence interval;
CRS/NP, chronic rhinosinusitis and/or nasal polyps;
CRS, chronic rhinosinusitis;
CRSwNP, chronic rhinosinusitis with nasal polyps;
EGPA, eosinophilic granulomatosis with polyangiitis;
EoE, eosinophilic esophagitis;
EOS, eosinophils;
ERS, European Respiratory Society;
FeNO, fraction of exhaled nitric oxide;
FEV1, Forced Expiratory Volume;
GINA, Global Initiative for Asthma;
ICS, inhaled corticosteroids;
IgE, immunoglobulin E;
IL, interleukin;
IL-5R, interleukin 5 receptor;
ILC, innate lymphoid cell;
ISAR, International Severe Asthma Registry;
IU, international units;
LABA, long-acting ß2-agonist;
LAMA, long-acting muscarinic antagonist;
LSM, least squares mean;
LTRA, leukotriene receptor antagonist;
M4C, Medicines for Children;
MCID, minimal clinically important difference;
MDT, multidisciplinary team;
MHRA, Medicines and Healthcare products Regulatory Agency;
MMRM, Mixed Models for Repeated Measures;
NHS, National Health Service;
NICE, National Institute for Health and Care Excellence;
NO, nitric oxide;
NP, nasal polyps;
OCS, oral corticosteroids;
OLE, open-label extension;
PACQLQ, Paediatric Asthma Caregiver’s Quality of Life Questionnaire;
PAQLQ(S)-IA, Paediatric Asthma Quality of Life Questionnaire with Standardised Activities-Interviewer Administered;
PAS, Patient Access Scheme;
PK, pharmacokinetics;
PN, prurigo nodularis;
ppb, parts per billion;
Q2W, every 2 weeks;
Q4W, every 4 weeks;
SC, subcutaneous;
SE, standard error;
SIGN, Scottish Intercollegiate Guidelines Network;
SMC, Scottish Medicines Consortium;
SmPC, Summary of Product Characteristics;
SNOT-22, Sino-Nasal Outcome Test-22;
SOC, standard of care;
TA, technology appraisal;
TdAP, tetanus, diphtheria and pertussis;
Th0, naïve T cell;
Th2, T helper type 2;
TSLP, thymic stromal lymphopoietin;
UK, United Kingdom.
1. DUPIXENT Summary of Product Characteristics. Great Britain. Date last accessed: February 2025.
2. Gandhi NA, et al. Nat Rev Drug Discov. 2016;15(1):35-50.
3. Robinson D, et al. Clin Exp Allergy. 2017;47(2):161-75.
4. Scottish Medicines Consortium. SMC2317 Dupilumab (Dupixent). 2021. Available at: https://scottishmedicines.org.uk/medicines-advice/dupilumab-dupixent-full-smc2317/ Date last accessed: February 2025.
5. IQVIA Sanofi Integrated DUPIXENT Platform, data through November 2024.
6. Pavord ID, et al. Lancet. 2018;391(10118):350-400.
7. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2024. Available at: https://ginasthma.org/wp-content/uploads/2024/05/GINA-2024-Strategy-Report-24_05_22_WMS.pdf Date last accessed: February 2025.
8. Le Floc'h A, et al. Allergy. 2020;75(5):1188-204.
9. Saatian B, et al. Tissue Barriers. 2013;1(2):e24333.
10. Peters MC, et al. J Allergy Clin Immunol. 2014;133(2):388-94.
11. Seys SF, et al. Respir Res. 2017;18(1):39.
12. Denton E, et al. J Allergy Clin Immunol Pract. 2021;9(7):2680-8 e7.
13. Castro M, et al. N Engl J Med. 2018;378(26):2486-96.
14. Sanofi UK. Data On File. High ICS and Type 2 severe asthma sub-analysis. REF-145318. August 2021.
15. Bacharier LB, et al. New England Journal of Medicine. 2021;385(24):2230-40.
16. Fleming L, et al. Eur Respir J. 2015;46(5):1322-33.
17. Nunes C, et al. Asthma Res Pract. 2017;31.
18. Bellin MH, et al. J Pediatr Health Care. 2015;29(6):536-46.
19. Sanofi UK. Data on file. LIBERTY ASTHMA VOYAGE. CSR. REF-205726. August 2020.
20. Rabe KF, et al. N Engl J Med. 2018;378(26):2475-85.
21. Sweeney J, et al. Thorax. 2016;71(4):339-46.
22. Sarnes E, et al. Clin Ther. 2011;33(10):1413-32.
23. Waljee AK, et al. BMJ. 2017;357j1415.
24. Sher LD, et al. Chest. 2022;162(1):46-55.
25. Wechsler ME, et al. Lancet Respir Med. 2022;10(1):11-25.
26. Bacharier LB, et al. Lancet Respir Med. 2024;12(1):45-54.
27. Scottish Medicines Consortium. SMC2598 Dupilumab (Dupixent). 2021. Available at: https://scottishmedicines.org.uk/medicines-advice/dupilumab-dupixent-full-smc2598/ Date last accessed: February 2025.
28. NICE. TA955 Dupilumab for treating moderate to severe prurigo nodularis. 2024. Available at: https://www.nice.org.uk/guidance/ta955 Date last accessed: February 2025.
29. DUPIXENT 200 mg solution for injection in pre-filled pen. Patient Information Leaflet. Date last accessed: February 2025.
30. DUPIXENT 200 mg solution for injection in pre-filled syringe. Patient Information Leaflet. Date last accessed: February 2025.
31. NICE. TA751 Dupilumab for treating severe asthma with Type 2 inflammation. 2021. Available at: https://www.nice.org.uk/guidance/TA955 Date last accessed: February 2025.
32. NHS England. Commissioning Medicines for Children in Specialised Services. 2017. Available at: https://www.england.nhs.uk/publication/commissioning-medicines-for-children-specialised-services/ Date last accessed: February 2025.
32. Northern Ireland Formulary. Managed Entry Decisions – DUPIXENT. Available at: https://niformulary.hscni.net/managed-entry/managed-entry-decisions/ Date last accessed: February 2025.
MAT-XU-2400904 (v2.0) Date of Preparation: February 2025