- Article
- Source: Campus Sanofi
- 28 Feb 2025
An Introduction to Atopic Dermatitis
What is Atopic Dermatitis
Atopic dermatitis (AD) is a chronic disease driven by persistent underlying inflammation even in nonlesional or normal looking skin 1-3
- Atopic dermatitis is an immunological disease where the immune system causes more inflammation than normal
- The overactive immune system under the surface may lead to increased inflammation on the surface and is a contributing factor to the itchy patches on the skin
- Moderate-to-severe AD in adults is a chronic, debilitating inflammatory condition that requires long-term control 1,4
Challenges of treating Atopic Dermatitis
Atopic dermatitis (AD) is a chronic disease driven by persistent underlying Type 2 inflammation 1-3
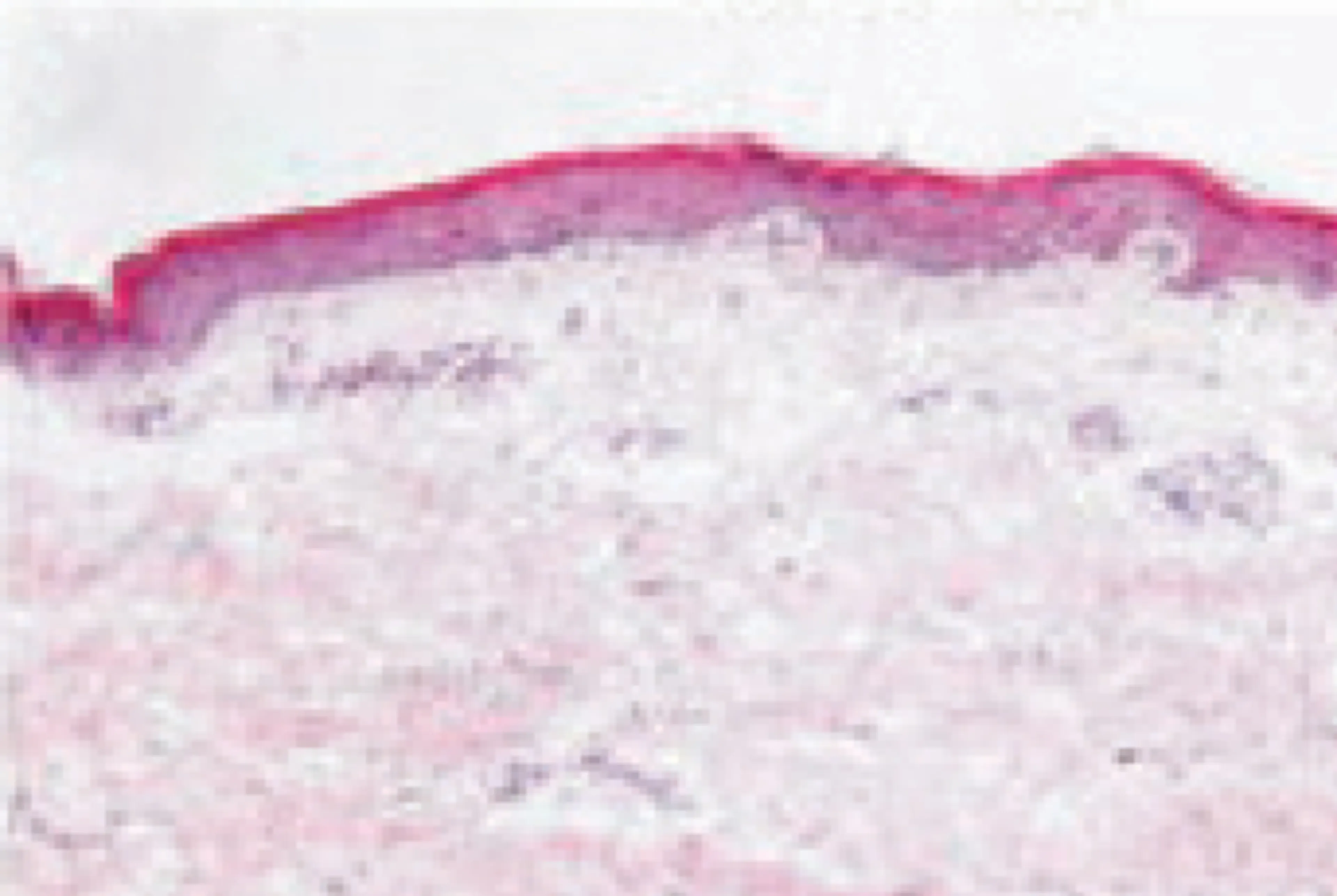
Even skin that appears non-lesional on the surface is clearly distinct from normal skin:1-3
Normal skin

Lesional skin

Non-lesional skin

Normal skin

Lesional skin

Non-lesional skin
Non-lesional skin in AD has been shown to have cutaneous expansion of T cells and abnormalities that are largely similar to lesional AD skin. This highlights the importance of early identification and treatment of patients with this condition.
- In the UK, ciclosporin is the only conventional systemic immunosuppressant approved for the management of severe AD in adults5. Other systemic immunosuppressants are used off-label, despite limited evidence supporting their efficacy and safety6-8
- Systemic immunosuppressants exert broad suppression of the immune system, while immunomodulators target specific immune pathways/cell types, thereby reducing off-target effects9
- Broad-acting immunosuppressants are associated with a range of toxicities and side effects that limit their long-term use and require routine laboratory monitoring to minimise the risks 6,7,10,11
Watch Dr. Woolf discuss the impact of immunosuppressant cycling:
AD experts* proposed four types of treatment failure which patients with AD may experience despite appropriate dose, duration and adherence to a therapeutic agent12:
- Inadequate clinical improvement12
- Failure to achieve stable long-terms disease control12
- Adverse events or poor tolerability with current treatment12
- Failure to relieve burden of disease† 12
*A steering committee that consisted of a multi-disciplinary group of AD experts, including 8 dermatologists, two allergists and a patient advocacy group representative. This group met to identify and prioritise a number of essential questions concerning the evaluation and management of moderate-to-severe AD in the era of biologic therapies
†Presence of ongoing impairment (e.g., pruritus, pain, loss of sleep, and poor quality of life) while on treatment
The Role of Type 2 Inflammation
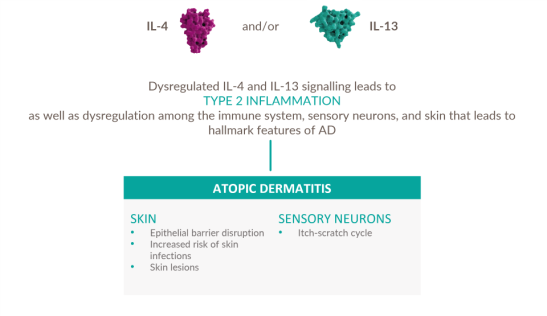
Dysregulated signaling of IL-4 and IL-13—two key drivers of type 2 inflammation—plays an important role in the pathogenesis of AD 13-18
IL-4 is a key upstream modulator of Th2 response, promoting a positive feedback loop that contributes to dysregulated secretion of IL-4, IL-13, and IL-31 #
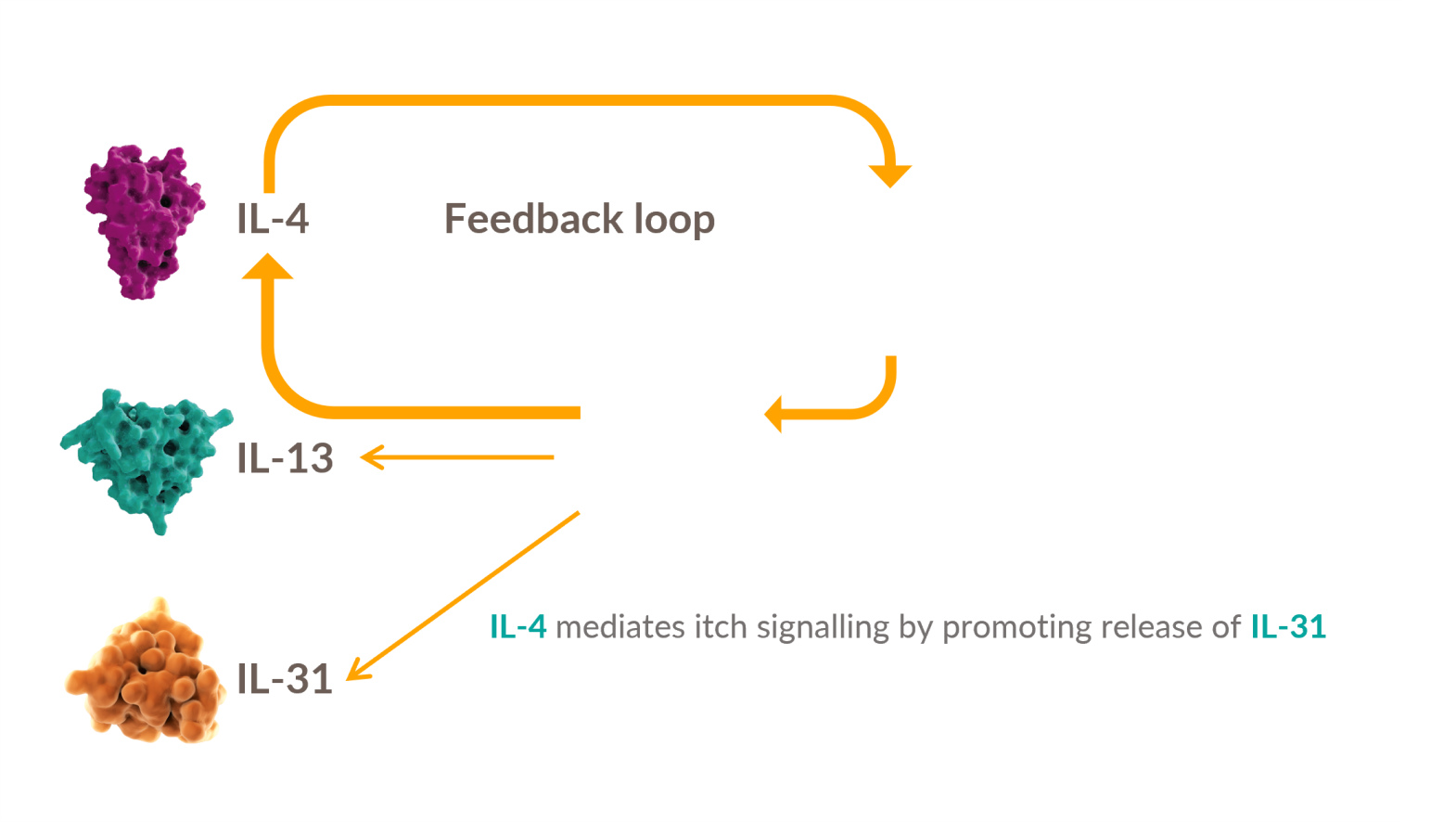
IL-4 and IL-13 have overlapping roles in Type 2 inflammation:
Watch Dr Alan Irvine discuss the role of IL-4 in Type 2 Inflammation for more information
Management approaches in AD should address the persistent underlying Type 2 inflammation
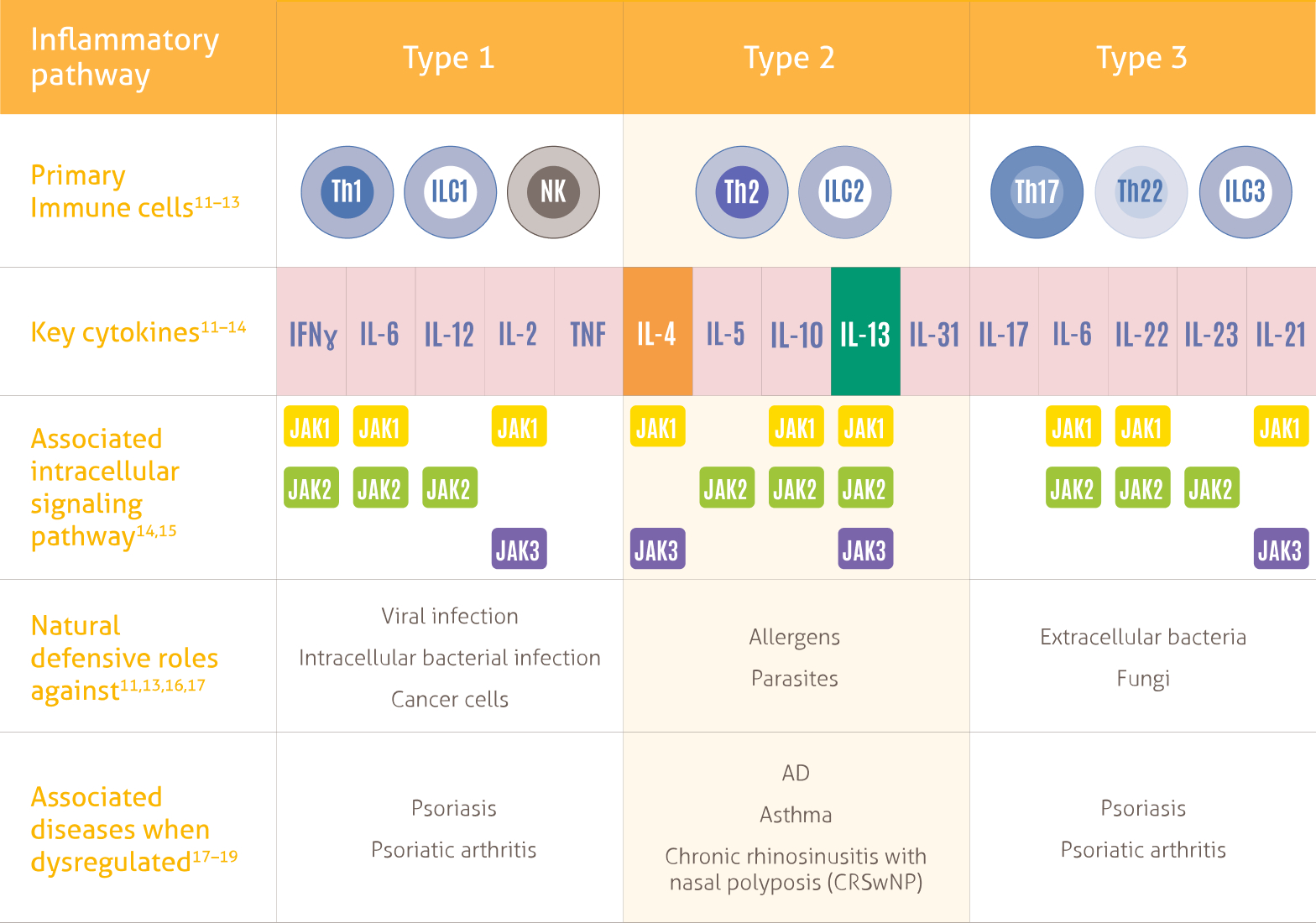
- Three types of immune response play a natural protective role against different pathogens, the Type 2 immune response drives AD specifically 1-3
- Broad spectrum immunosuppressants do not specifically inhibit Type 2 inflammatory pathway 19-21
- Non-specific mechanisms may result in unwanted side effects and the need for lab monitoring, limiting traditional systemic treatments as long-term therapeutic options 1,19-24
Watch this video to hear Dr Woolf discussing the immunological targets at play in AD:
DUPIXENT directly targets the underlying type 2 inflammation involved in AD.
References
- Leung DYM, et al. J Clin Invest. 2004;113(5):651–657.
- Suárez-Fariñas M, et al. J Allergy Clin Immunol. 2011;127(4):954–964
- Gittler JK, et al. J Allergy Clin Immunol. 2012;130(6):1344–1354.
- Huang A, et al. Curr Treat Options Allergy. 2017;4(3):355–369
- Neoral Soft Gelatin Capsules. Summary of Product Characteristics. Available at https://www.medicines.org.uk/emc/product/1034/smpc Date last accessed: February 2025.
- Wollenberg A, et al. J Eur Acad Dermatol Venereol. 2018;32(6):850-878
- Wollenberg A, et al. J Eur Acad Dermatol Venereol. 2022;36(9):1409-1431
- Bieber T, Straeter B. Allergy. 2015;70:6–11.
- Janssen M, et al. Intensive Care Med. 2023;49(4):462-4
- Berth-Jones J, et al. Br J Dermatol 2019;180(6):1312–38
- Armstrong AW, et al. PLoS ONE. 2019;14(1):e0210517
- Boguniewicz M, et al. J Allergy Clin Immunol Pract. 2017;5:1519–1531.
- DUPIXENT Summary of Product Characteristics. 2023
- Serezani APM et al. J Allergy Clin Immunol 2017;139(1):142-151.
- Silverberg JI et al. Dermatol Clin 2017;35(3):327-334
- Nguyen JK et al. Arch Dermatol Res 2020;312(2):81-92.
- Haddad E-B et al. Dermatol Ther (Heidelb) 2022;12(7):1501-1533
- Oetjen LK et al. Cell 2017;171(1):217-228
- Hajar T, et al. An Bras Dermatol. 2018;93:104–107
- Wakelin SH, et al. Medicine. 2017;45:363–367
- Moyle M, et al. Exp Dermatol. 2019;28:756–768
- Seegräber M, et al. Expert Rev Clin Pharmacol. 2018; 11:467–474
- Armstrong AW, et al. PLoS ONE. 2019:14:e0210517
- Wu J et al. Expert Opin Biol Ther. 2020;20:525–538
- Kaiko GE, et al. Immunol. 2008;123:326–338.
- Eyerich K et al. J Eur Acad. 2017;32:692–703.
- Raphael I, et al. Cytokine. 2015;74:5–17
- Schwartz DM, et al. Nat Rev Rheumatol. 2016;12(1):25–36
- Murray PJ. J Immunol. 2007;178(5):2623–2629
- Annunziato F, et al. J Allergy Clin Immunol. 2015;135(3):626–635
- Schleimer R. Annu Rev Pathol. 2017;12:331–357
- Nakayama T, et al. Annu Rev Immunol. 2017;35:53–84.
- Coates LC, et al. Semin Arthritis Rheum. 2016;46:291–304

Atopic Dermatitis Control Test (ADCT)
Atopic dermatitis, a type of eczema, may be affecting your patient’s life in more ways than you know.
The ADCT gives a measure of how controlled your patient’s eczema is. Use these 6 concise questions to evaluate all dimensions of atopic dermatitis control.
Development of ADCT involved literature review as well as interviews with patients and physicians, and was funded by Sanofi and Regeneron.
Try the ADCTMAT-XU-2304408 (v2.0) Date of Preparation February 2025