.jpg)
Key Benefits of DUPIXENT
DUPIXENT is indicated for use in patients aged 12 years and older with moderate-to-severe atopic dermatitis (AD), and in patients aged 6 months and older with severe AD who are candidates for systemic therapy.1
DUPIXENT is the first and only targeted immunomodulator to target two key mediators of type 2 inflammation, responsible for atopic dermatitis - IL-4 and Il-13.1-3
Rapid and sustained control - Consistent across all licensed ages
- Sustained improvement of itch, skin clearance and quality of life (QoL) up to 16 weeks in infants, children and adolescents, and 52 weeks in adults, with rapid control 2 weeks after the first dose in adults and children, and 4 weeks in adolescents and infants vs placebo 1,4-17
Consistent long-term safety profile
- With safety data based on a 5-year clinical trial and in >750,000 patients worldwide, across all indications 8,18
- Approved in patients as young as 6 months old1
Start with ease, stay with confidence
- DUPIXENT requires no initial lab testing or ongoing monitoring as per the SmPC 1
Rapid and sustained control - Consistent across all licensed ages
- Sustained improvement of itch, skin clearance and quality of life (QoL) up to 16 weeks in infants, children and adolescents, and 52 weeks in adults, with rapid control 2 weeks after the first dose in adults and children, and 4 weeks in adolescents and infants vs placebo 1,4-17
Consistent long-term safety profile
- With safety data based on a 5-year clinical trial and in >750,000 patients worldwide, across all indications 8,18
- Approved in patients as young as 6 months old1
Start with ease, stay with confidence
- DUPIXENT requires no initial lab testing or ongoing monitoring as per the SmPC 1
Efficacy

Rapid and Sustained Control Across all licensed ages
Sustained improvement of itch, skin clearance, QoL and AD severity up to 16 weeks in infants, children and adolescents and, 52 weeks in adults. vs placebo.4,9,12,14,15,17 Rapid relief of itch and increased skin clearance and QoL 2 weeks after the first dose in adults and children and 4 weeks in adolescents and infants vs placebo.4-7,9-17
Individual Efficacy Outcomes
Adults: 18+ years at Week 16 and Week 52
Rapid and sustained improvements in skin clearance after the first dose (18+ years)
Proportion of patients achieving EASI-75 from baseline to Week 524,5
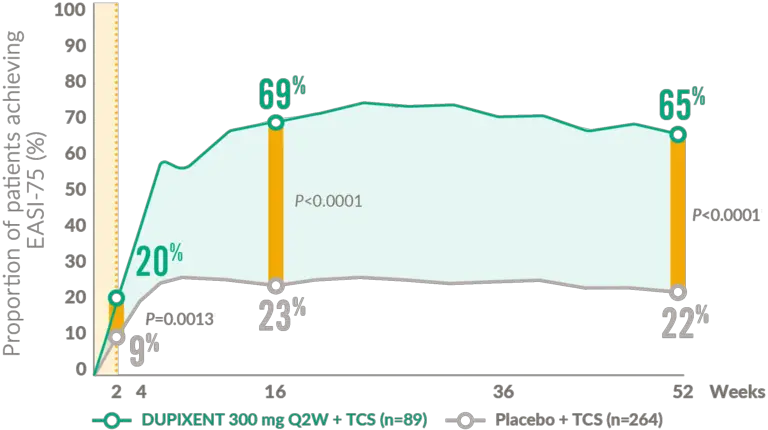
39 % of patients on DUPIXENT achieved IGA score of 0 or 1 with a reduction of ≥2 points on a 0–4 IGA scale compared to 12% of patients on placebo, at 16 weeks p<0.00014
EASI: eczema area and severity index; IGA: Investigator’s Global Assessment; Q2W: every 2 weeks; TCS: topical corticosteroids.
Rapid and sustained improvement in itch after the first dose (18+ years)
Mean Percentage improvement in pruritus NRS score from baseline to Week 524,6
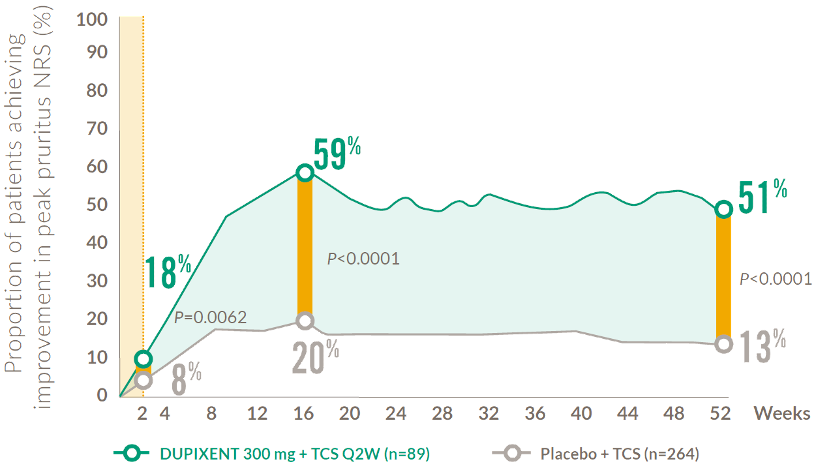
A clinically meaningful ≥4-point improvement was seen as early as Week 2.
*A clinically meaningful improvement in pruritus is defined as a ≥4-point improvement in pruritus NRS (0–10)
NRS: numerical rating scale; Q2W: every 2 weeks; TCS: topical corticosteroids.
Rapid and sustained improvement in quality of life after the first dose (18+ years)
Patients achieving clinically meaningful improvement* in DLQI from baseline to Week 524,19
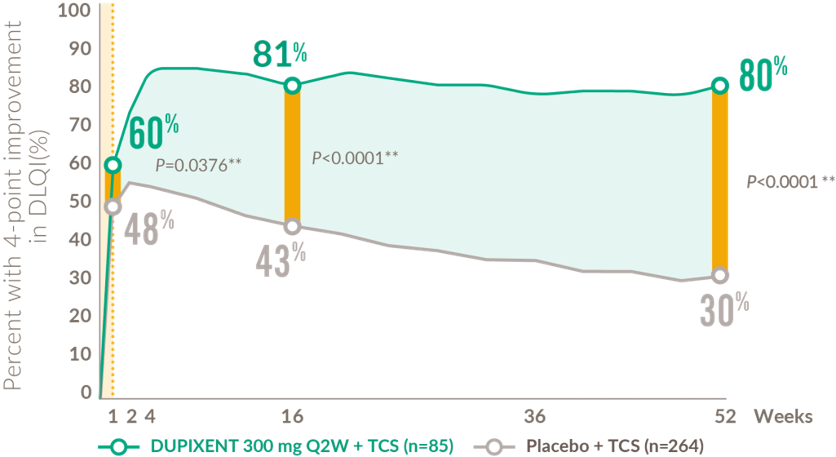
DLQI: dermatology quality of life index; Q2W: every 2 weeks; TCS: topical corticosteroids.
*Defined as a ≥4-point improvement in DLQI on a 0–30 point scale. Results from post-hoc analyses.
** Nominal p-value
- CHRONOS trial design: A randomised, double-blind, placebo-controlled study of adults with moderate-to-severe atopic dermatitis with prior inadequate response to TCS. 319 patients were randomised to DUPIXENT 300 mg + TCS QW, 106 patients were randomised to DUPIXENT 300 mg + TCS Q2W (licensed dose) and 315 patients were randomised to placebo + TCS for 52 weeks. Emollient background regimen/therapy was required during the trial.4
- The co-primary endpoints were the proportion of patients with both IGA 0/1 (clear/almost clear) and a ≥2-point reduction from baseline at Week 16 (0–4 scale), and the proportion of patients achieving 75% improvement in EASI (EASI-75) from baseline to Week 16.
- Other evaluated outcomes included: the proportion of patients with improvement of at least 50% and 90% in EASI (EASI-50 and EASI-90, respectively), reduction in itch as measured by the peak pruritus Numerical Rating Scale(NRS), percent change in the SCORing Atopic Dermatitis(SCORAD) scale from baseline to Week 16, mean change from baseline to Week 16 in the Patient Oriented Eczema Measure(POEM), Dermatology Life Quality Index (a clinically meaningful improvement in DLQI is defined as a ≥4-point improvement on a 0 to 30 scale), and Hospital Anxiety and Depression Scale(HADS) scores. Efficacy was also evaluated at Week 52.
Adolescents: 12-17 years at Week 16
Rapid and sustained improvements in skin clearance after the first dose (12-17 years)
Proportion of patients achieving EASI-75 from baseline to Week 1614,15
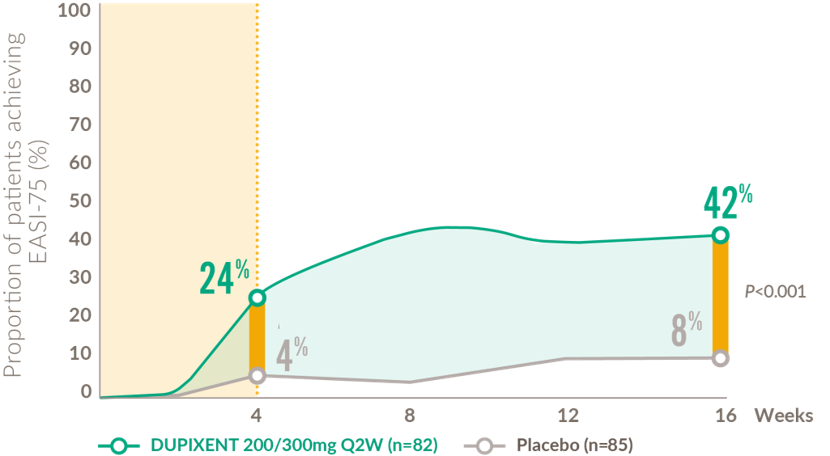
24% of patients on DUPIXENT achieved an IGA score of 0 or 1 compared to 2% of patients on placebo, at 16 weeks (P<0.001)13,22
EASI: eczema area and severity index; IGA: Investigator’s Global Assessment; Q2W: every 2 weeks.
Rapid and sustained improvements in itch after the first dose (12-17 years)
Proportion of adolescent patients achieving a clinically meaningful* improvement in peak pruritus NRS at Week 1613,14
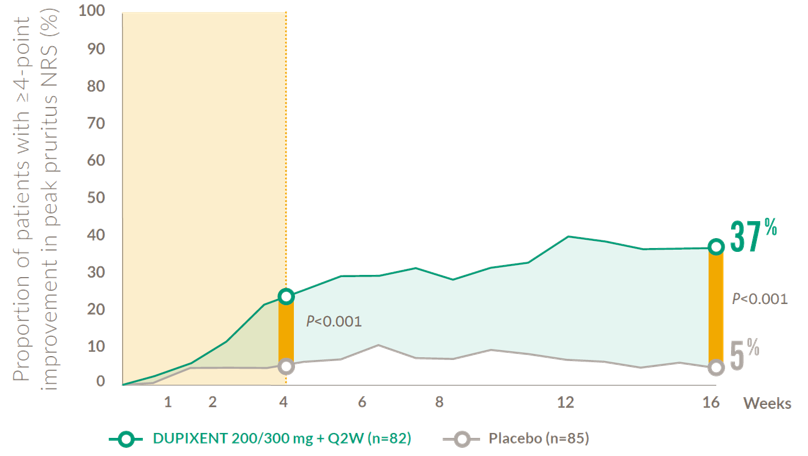
NRS: numerical rating scale; Q2W: every 2 weeks.
* A clinically meaningful improvement in pruritus is defined as a ≥4-point improvement in pruritus NRS (0–10)
Rapid and sustained improvements in quality of life after the first dose (12-17 years)
Patients achieving clinically meaningful improvement* in CDLQI from baseline to Week 1616
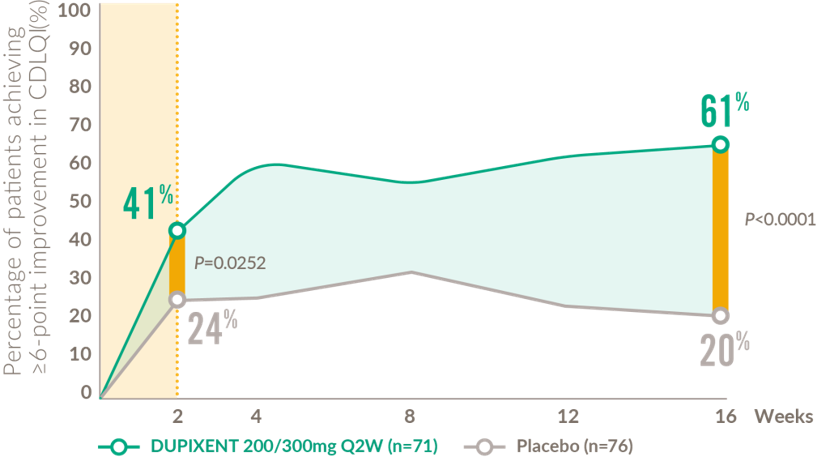
CDLQI: children’s dermatology quality of life index; Q2W: every 2 weeks.
*Defined as a ≥6-point improvement in CDLQI on a 0–30 point scale
- AD-1526: A randomised, placebo-controlled, double-blind, parallel-group, multicentre, Phase 3 trial of adolescent patients (n=251) with moderate-to-severe AD whose disease was inadequately controlled by topical treatment, randomised to receive either a) 200/300 mg DUPIXENT Q2W (n=82) (licensed dose), b) 300 mg DUPIXENT Q4W (n=84) or c) placebo Q2W for 16 weeks.
- The co-primary endpoints were the proportion of patients with both IGA 0/1 (clear/almost clear) and the proportion of patients achieving 75% improvement in EASI (EASI-75) from baseline to Week 16.
- The key secondary endpoints were the percentage change in EASI from baseline to Week 16, the percentage change in peak pruritus NRS score from baseline, proportion of patients with ≥3-point improvement in peak pruritus NRS score from baseline to Week 16, proportion of patients with ≥4-point improvement in peak pruritus NRS score from baseline to Week 16.
- Other secondary endpoints were the proportion of patients achieving at least 50% improvement in EASI (EASI-50), change from baseline in percent body surface area affected (BSA) by AD, percentage change from baseline in SCORing Atopic Dermatitis (SCORAD), and changes from baseline in Children’s Dermatology Life Quality Index (a clinically meaningful improvement in CDLQI is defined as a ≥6-point improvement on a 0–30 scale), Patient-Oriented Eczema Measure (POEM) and SCORAD sleep loss scores from baseline to Week 16.
Children: 6-11 years at Week 16
Rapid and sustained improvements in skin clearance after the first dose (6-11 years)
Proportion of patients achieving EASI-75 from baseline to week 169,10
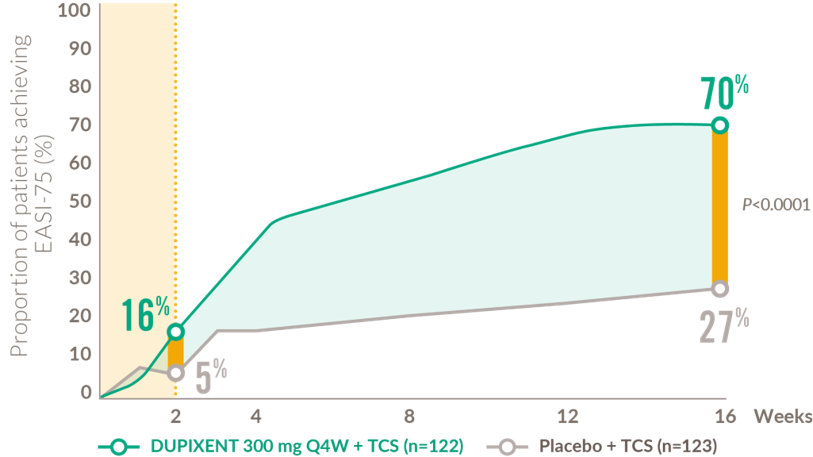
33% of patients on DUPIXENT + TCS achieved an IGA score of 0 or 1 compared to 11% of patients on placebo + TCS, at 16 weeks (P<0.0001)9
EASI: eczema area and severity index; IGA: Investigator’s Global Assessment; Q4W: every 4 weeks; TCS: topical corticosteroids.
Rapid and sustained improvements in itch after the first dose (6-11 years)
Proportion of child patients achieving a clinically meaningful* improvement in peak pruritus NRS at Week 169
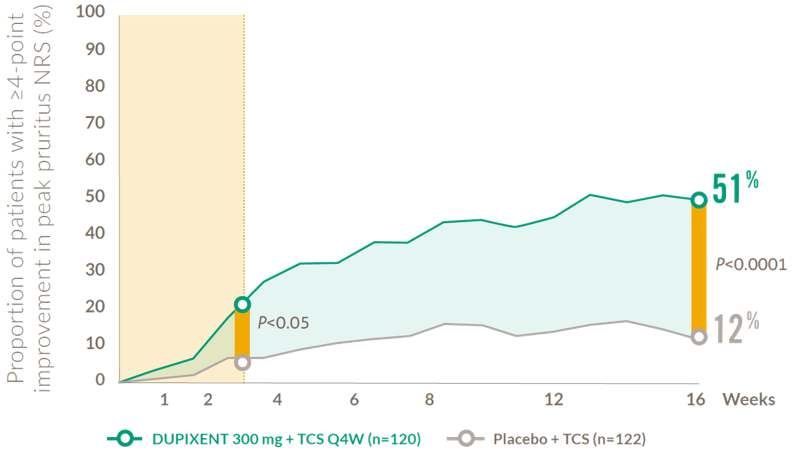
*Defined as a ≥4-point improvement in NRS on a 0–10 point scale
NRS: numerical rating scale; Q4W: every 4 weeks; TCS: topical corticosteroids.
Rapid and sustained improvement in quality of life after the first dose (6-11 years)
Patients achieving clinically meaningful improvement* in CDLQI from baseline to Week 1612
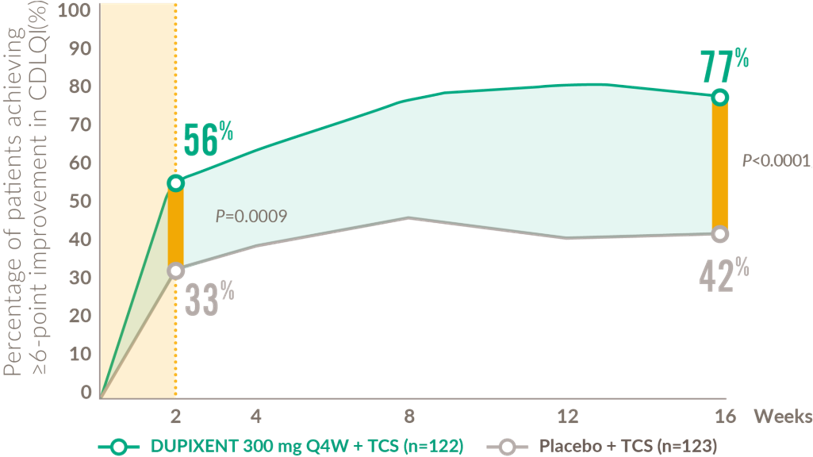
*Defined as a ≥6-point improvement in CDLQI on a 0–30 point scale
CDLQI: children’s dermatology quality of life index; Q4W: every 4 weeks.
- AD-1652: A randomised (1:1:1), placebo-controlled, double-blind, parallel-group, Phase 3 trial of child patients aged 6–11 years (N=367) with severe AD whose disease was inadequately controlled by topical treatment, randomised to receive either a) 300 mg DUPIXENT Q4W + TCS (n=122), b) 100 mg or 200mg DUPIXENT Q2W + TCS (n=122) or c) placebo + TCS (n=123) for 16 weeks.
- The co-primary endpoints were the proportion of patients with IGA 0 or 1 at Week 16 and the proportion of patients with EASI-75 at Week 16.
- The key secondary endpoints were the percentage change from baseline in EASI at Week 16 and the percentage change in weekly average of daily peak pruritus NRS.
- Other secondary endpoints were proportion of patients with ≥3-point improvement in peak pruritus NRS, proportion of patients with ≥4-point improvement in peak pruritus NRS, percentage of patients with EASI-50 and EASI-90, percentage change in SCORAD, change in CDLQI and POEM, change from baseline in PROMIS patient anxiety and depression and SCORAD sleep loss scores from baseline.
Infants 6 months – 5 years at Week 16
Efficacy data is from a post-hoc analysis of total population of LIBERTY AD PRESCHOOL containing only patients with severe AD, in line with the DUPIXENT licence.
Rapid and sustained improvements in skin clearance after the first dose* (6 months -5 years)
Proportion of patients achieving EASI-75 from baseline to Week 1617
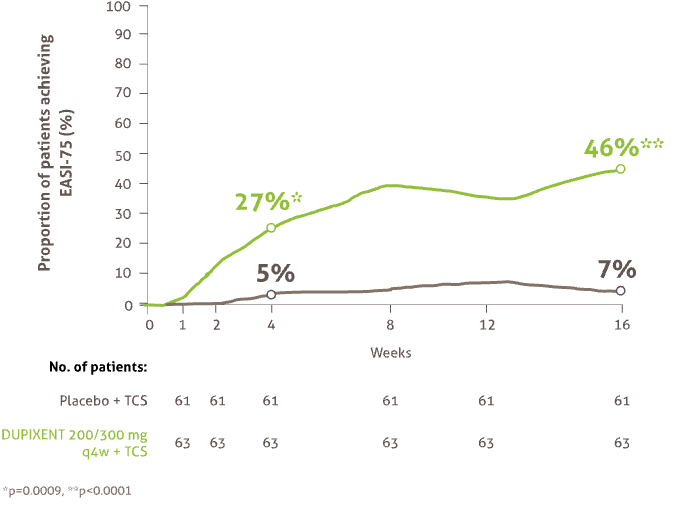
43% of patients on DUPIXENT + TCS achieved an IGA score of 0 or 1 vs 8% on placebo + TCS at 16 weeks (p<0.0001)17
* Efficacy data is from a post-hoc analysis of a subset of the total population of LIBERTY AD PRESCHOOL containing only patients with severe AD, in line with DUPIXENT licence17.
AD : atopic dermatitis EASI : Eczema Area and Severity Index IGA : Investigator’s Global Assessment Q4W : every 4 weeks; TCS : topical corticosteroids
Rapid and sustained improvements in itch after the first dose* (6months -5 years)
Mean percentage change in worst scratch/itch NRS score from baseline to Week 1617
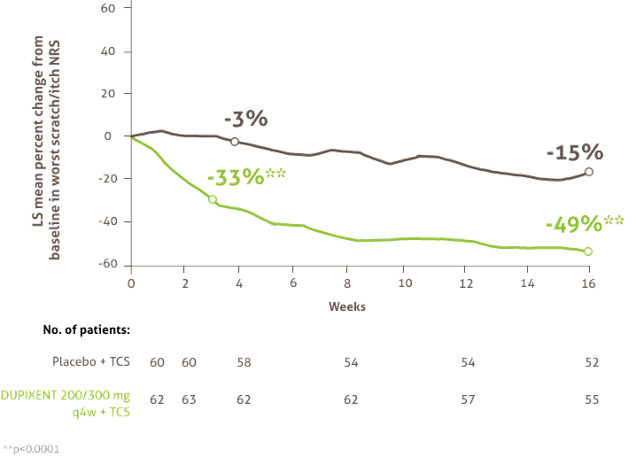
* Efficacy data is from a post-hoc analysis of a subset of the total population of LIBERTY AD PRESCHOOL containing only patients with severe AD, in line with DUPIXENT licence17.
AD, atopic dermatitis; NRS, Numerical Rating Score; Q4W, every 4 weeks; TCS, topical corticosteroids.
Improvement in quality of life at week 16* (6 months - 5 years)
Mean change from baseline in IDQOL and CDLQI at Week 1617
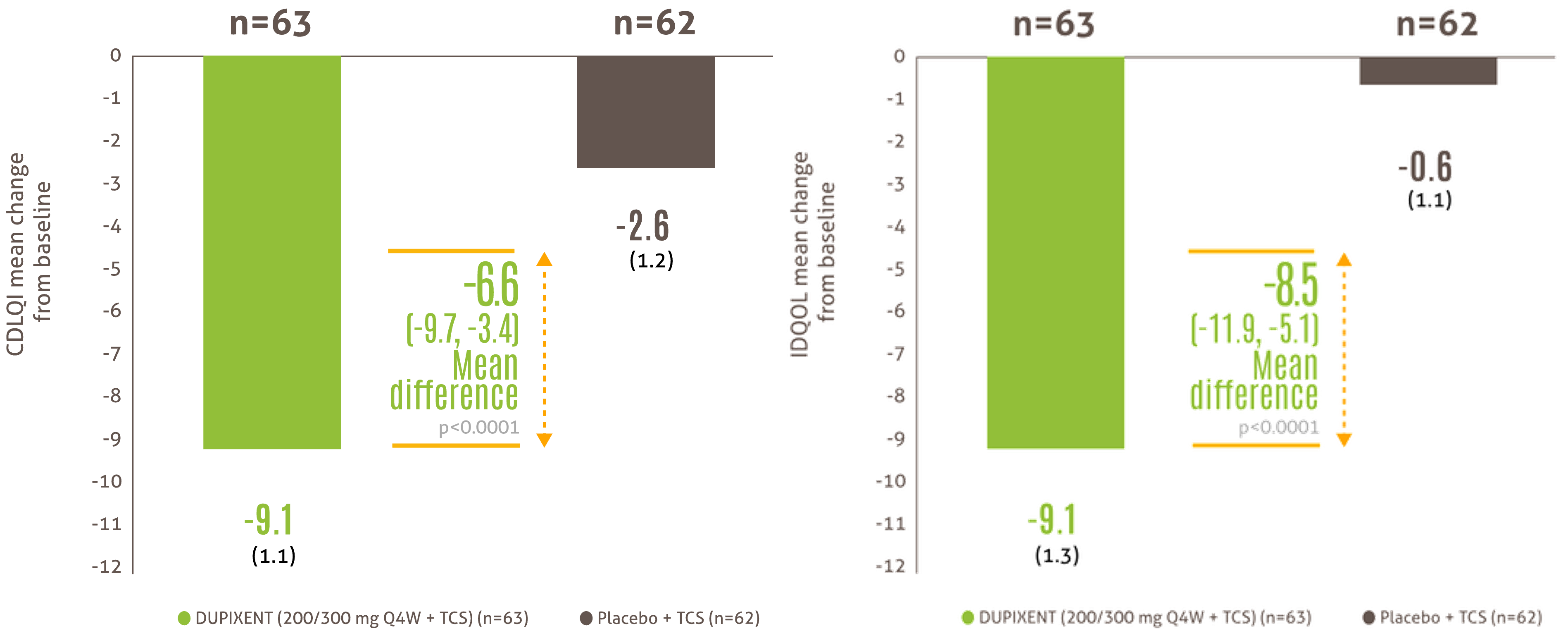
Patients between 6 months and 5 years old on DUPIXENT + TCS achieved a clinically meaningful improvement in quality of life compared to patients on placebo + TCS, at 16 weeks
IDQOL was measured in patients <4years of age. CDLQI was measured in patients aged ≥4 to <6 years. Clinically meaningful defined as a ≥6-point improvement in IDQOL or CDLQI score.
* Efficacy data is from a post-hoc analysis of a subset of the total population of LIBERTY AD PRESCHOOL containing only patients with severe AD, in line with DUPIXENT licence17.
AD, atopic dermatitis; CDLQI, Children’s Dermatology Quality of Life Index; IDQOL, Infants’ Dermatitis Quality of Life Index; Q4W, every 4 weeks; SE, standard error; TCS, topical corticosteroids.
- A randomised (1:1), placebo-controlled, double-blind, parallel-group, Phase 3 trial of infant patients aged 6 months to 5 years (N=162) with inadequately controlled moderate-to-severe AD. Data presented above are from the post hoc analysis in infants with severe AD. Patients were randomised to either 200/300 mg DUPIXENT every 4 weeks (200 mg if baseline weight ≥5 to <15 kg, 300 mg if ≥15 to <30 kg) + TCS or placebo + TCS for 16 weeks.
- The co-primary endpoints were the proportion of patients with Eczema Area and Severity Index (EASI)-75 (≥75% improvement from baseline) at Week 16 and the proportion of patients with an IGA score of either 0 or 1 (on a 5-point scale) at Week 16. These were met.
- The key secondary endpoints were the percent change in EASI score from baseline to Week 16, and the percent change from baseline to Week 16 in weekly average of daily worst scratch/itch Numeric Rating Scale (NRS) score. These were met.
DUPIXENT was studied specifically in adults and adolescents with moderate-to-severe hand and foot atopic dermatitis
Rapid and significant improvements in clinical outcomes and QoL with DUPIXENT vs placebo
Statistically significant improvements in skin clearance, itch, QoL and AD severity were achieved at 16 weeks in adults and adolescents with hand and foot atopic dermatitis (HF AD) vs placebo.23
Rapid improvements vs placebo were observed as early as week 1 for itch and week 2 for skin clearance and QoL (nominally significant).23
The safety profile was consistent with the known safety profile of DUPIXENT.23
Efficacy Outcomes
Adults and adolescents at week 16
More than 2 x as many adult and adolescent patients taking DUPIXENT achieved skin clearance at week 16 vs placebo
Proportion of patients achieving hand and foot IGA 0/1 over time23
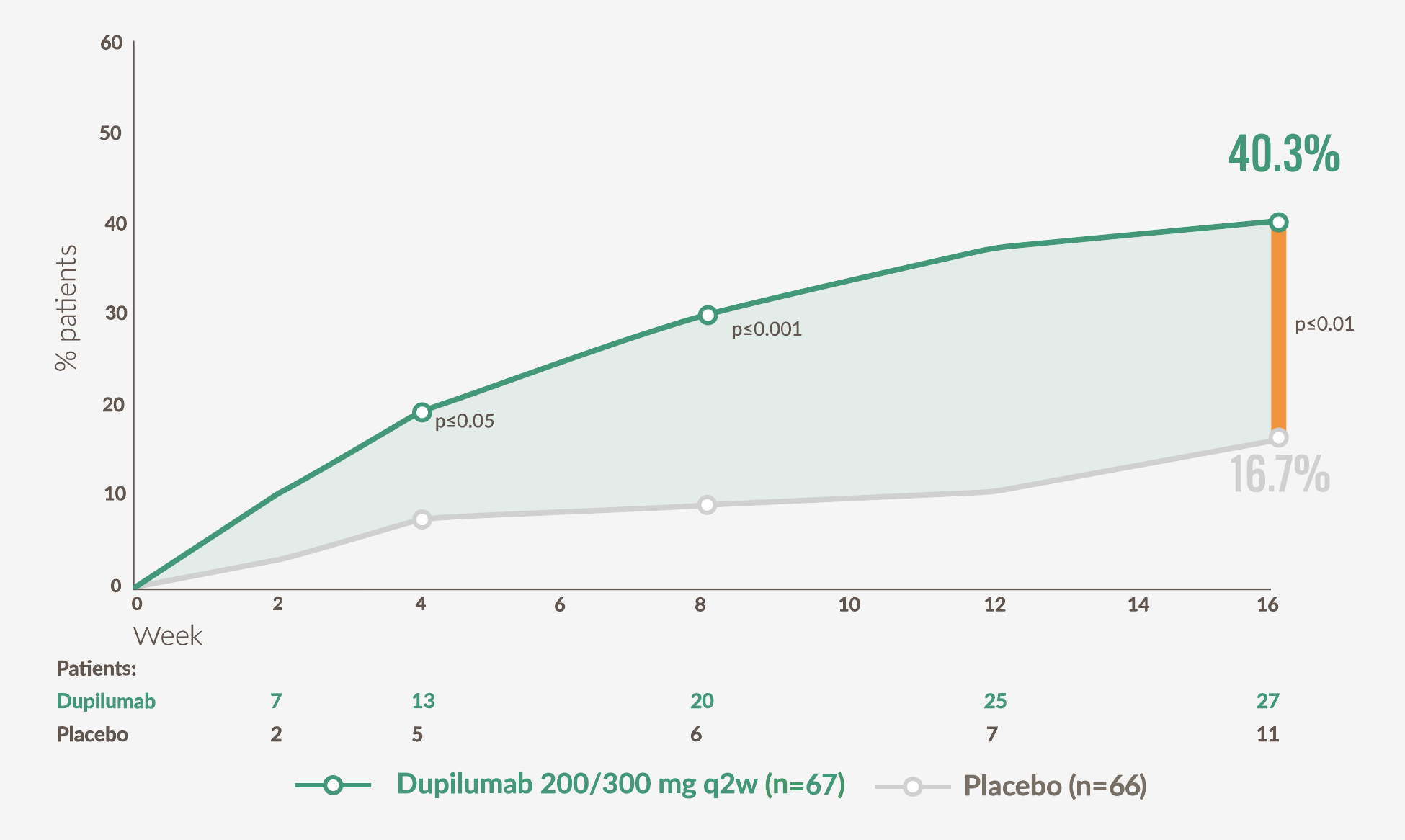
Adapted from Simpson EL, et al. 2024.23
40.3% of DUPIXENT patients achieved IGA score of 0 or 1 compared to 16.7% of patients on placebo at 16 weeks p≤0.01.23
IGA, Investigator’s Global Assessment; Q2W, every 2 weeks.
Approximately 4 x as many patients taking DUPIXENT achieved itch improvement at week 16 vs placebo
Proportion of patients achieving a ≥4-point improvement in weekly average of daily hand and foot PP-NRS over time23
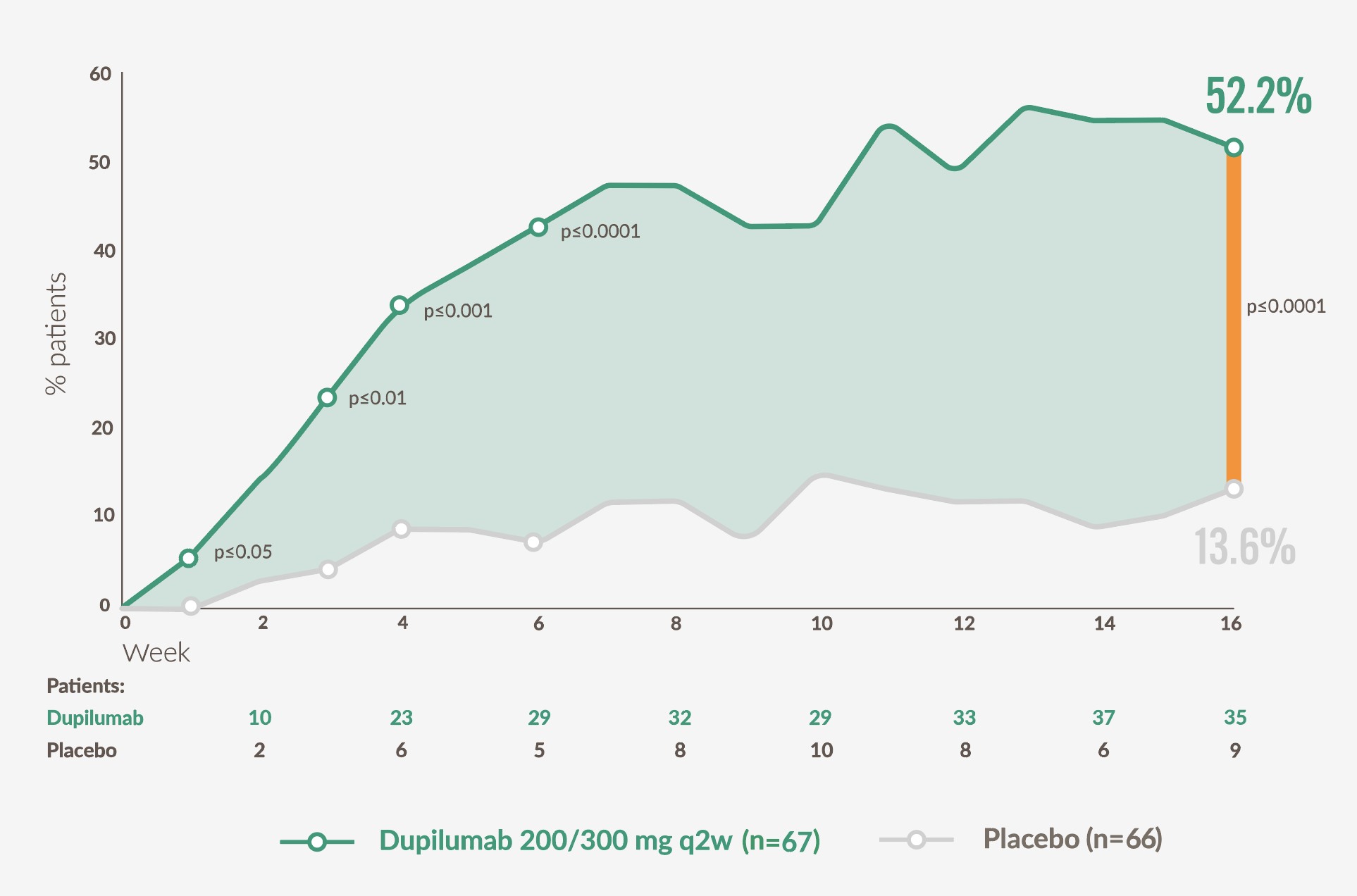
Adapted from Simpson EL, et al. 2024.23
52.2% of DUPIXENT patients achieved ≥4-point reduction in HF-Peak Pruritus NRS from baseline compared to 13.6% of patients on placebo at week 16 (p<0.0001), with nominally significant improvements observed as early week 1 (p≤0.05).23
HF, hand and foot; NRS Numerical Rating Scale; PP-NRS, Peak Pruritis Numerical Rating Scale; Q2W, every 2 weeks.
More than 2 x as many patients taking DUPIXENT achieved significant improvement in QoLHEQ at week 16 vs placebo
Mean change from baseline in QoLHEQ over time24
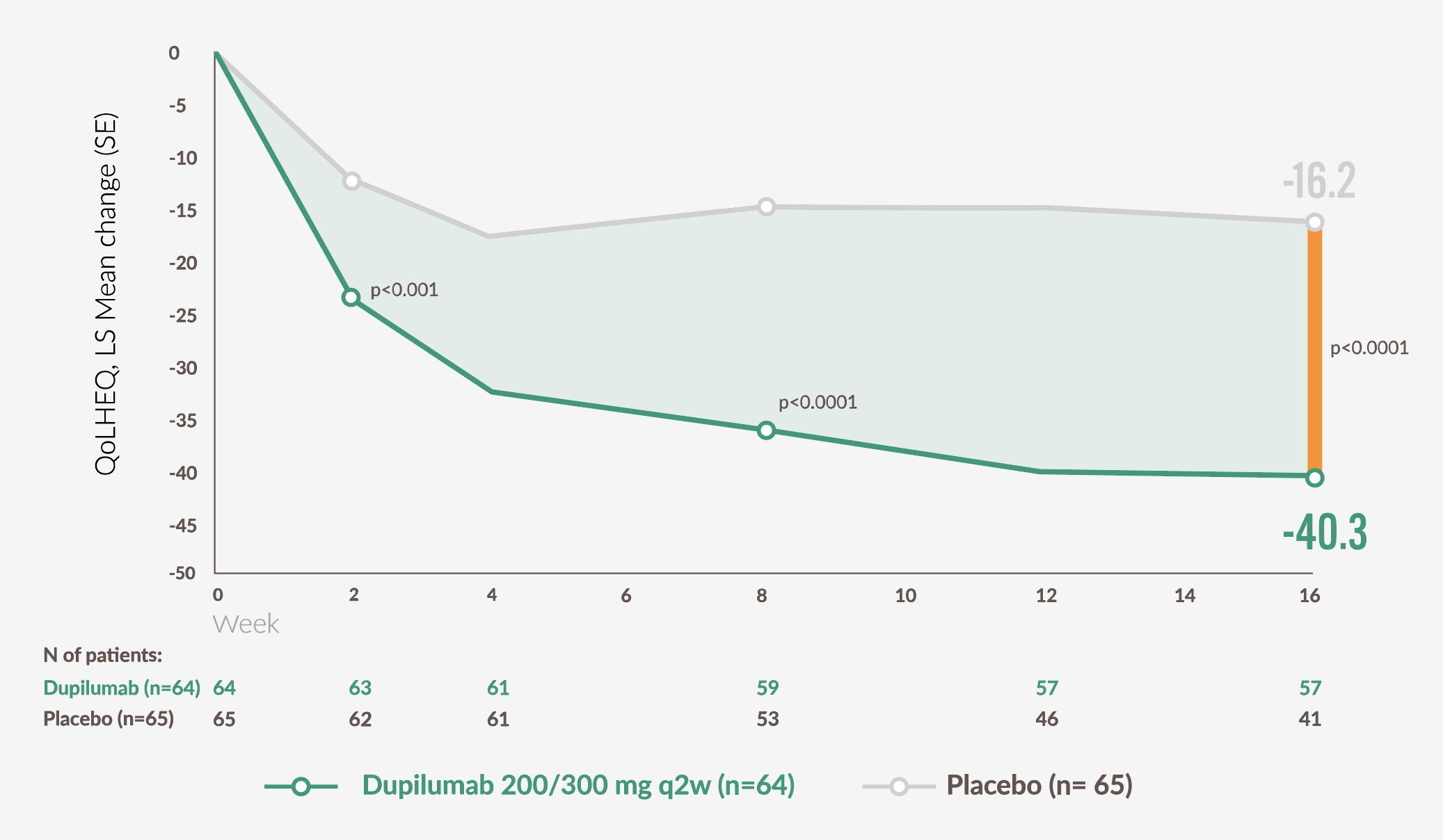
Adapted from Simpson EL, et al. 2024 [suppl].24
DUPIXENT patients achieved a significant improvement from baseline compared to patients on placebo at week 16 p<0.0001 (40.3 vs 16.2), with nominally significant improvements observed as early as week 2 (p<.001).23,24
LS, least squares; QoLHEQ, Quality of Life in Hand Eczema Questionnaire; Q2W, every 2 weeks; SE, standard error.
- LIBERTY-AD-HAFT trial design: A, randomised (1:1), double-blind, placebo-controlled Phase 3 study in adults aged 18 years and older (N=106) and adolescents aged 12–18 years (N=27) with moderate-to-severe HF AD. Patients were randomised to receive either DUPIXENT 300/200 mg (N=67) or placebo (N=66) every 2 weeks for 16 weeks.
- The key primary endpoint was proportion of patients achieving HF-IGA score 0 or 1 at week 16.
- The key secondary endpoint was the proportion of patients with ≥ 4-point reduction in HF-Peak Pruritus NRS from baseline at week 16.
- Other secondary endpoints were percentage change from baseline in mTLSS for H/F lesions, percentage change from baseline in weekly average of daily maximum HF-Peak Pruritus NRS score, change from baseline in weekly average of daily maximum HF-Skin Peak Pain NRS score, percentage change from baseline in HECSI score, proportion of patients with HECSI-75, change from baseline in percent surface area of H/F involvement with AD, change from baseline in QoLHEQ and change from baseline in weekly average of daily Sleep NRS score, at week 16.
AD, atopic dermatitis; HF, hand and foot; H/F, hand and/or foot; HECSI, Hand Eczema Severity Index; HF-IGA, Hand and Foot Investigator’s Global Assessment; mTLSS, modified Total Lesion Sign Score; NRS, Numerical Rating Score.
DUPIXENT Patient Case Study
Visible results demonstrated in an adult patient at Week 16 with DUPIXENT monotherapy
Baseline
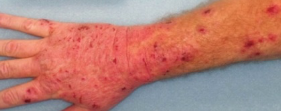
Week 16
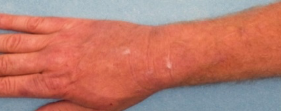
Clinical photographs are of an actual patient treated with DUPIXENT in the SOLO trial and are used with patient consent. Individual results may vary. SOLO 1 and 2 were two randomised, placebo-controlled trials of identical design (SOLO 1, n=671 and SOLO 2, n=708) in adults with moderate-to-severe atopic dermatitis (n=740), randomised to DUPIXENT 300 mg or placebo for 16 weeks.
Co-primary endpoints were the proportion of patients achieving EASI-75 (48% of patients treated with DUPIXENT vs 13% with placebo), and an IGA score of 0 or 1 with a reduction from baseline of ≥2 points at Week 16 (37% of patients treated with DUPIXENT vs 9% with placebo)25
- SOLO 1 and 2 trial design: Two randomised, placebo-controlled trials of identical design (SOLO 1, n=671 and SOLO 2, n=708) of adults with moderate-to-severe atopic dermatitis whose disease was inadequately controlled by topical treatment, randomized to receive either DUPIXENT 300 mg QW, DUPIXENT 300 mg Q2W (licensed dose) or placebo for 16 weeks. Emollient background regimen/therapy was required during the trial.
- The co-primary endpoints were the proportion of patients with both IGA 0/1 (clear/almost clear) and 2 point or higher reduction from baseline at Week 16 (0–4 scale), and the proportion of patients achieving 75% improvement in EASI (EASI-75) from baseline to Week 16.
- Other evaluated outcomes included: the proportion of patients with improvement of at least 50% and 90% in EASI score (EASI-50 and EASI-90, respectively) at Week 16, reduction in itch as measured by the peak pruritus Numerical Rating Scale (NRS) from baseline to Week 16, percent change in the SCORing Atopic Dermatitis (SCORAD) scale from baseline to Week 16, mean change in the Patient Oriented Eczema Measure (POEM) from baseline to Week 16, Dermatology Life Quality Index (DLQI – a clinically meaningful improvement is defined as a ≥4-point improvement on a 0–30 scale), and Hospital Anxiety and Depression Scale (HADS) scores.
Watch Dr Woolf (Guy's and St Thomas Hospital) talk about Mark's story: a 48 year-old man with long-term AD which heavily burdened his quality of life, and the impact treatment with DUPIXENT has had on his AD:
DUPIXENT Real world evidence
- Dupixent has been studied in adult patients (≥18 years) with moderate to severe AD in several real world UK settings27-31
- Efficacy results are similar to interventional Phase 3 clinical trials
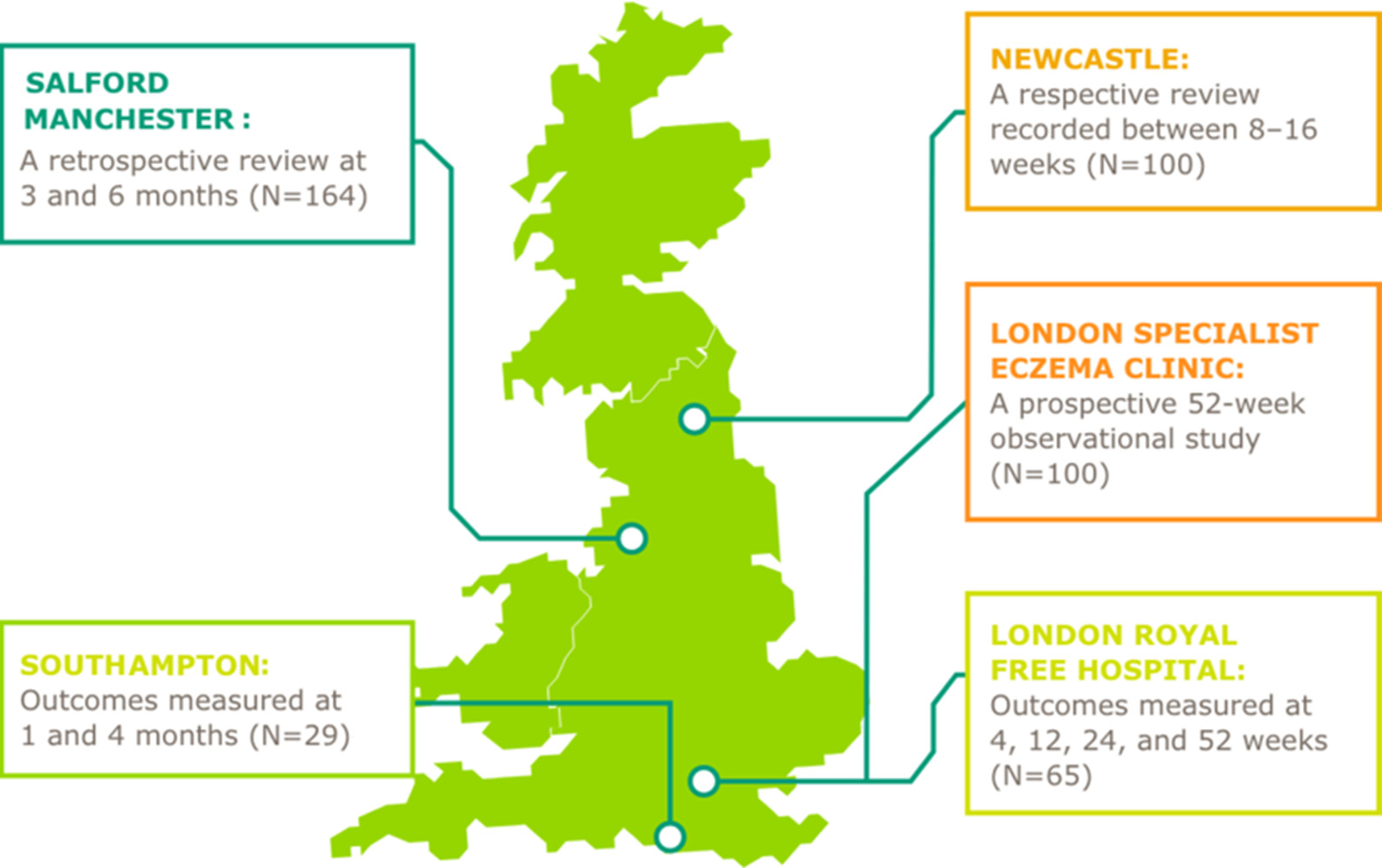
| City | Results |
| LONDON ROYAL FREE HOSPITAL31 |
|
| LONDON SPECIALIST ECZEMA CLINIC29 |
|
| MANCHESTER (SALFORD)28 |
|
| NEWCASTLE27 |
|
| SOUTHAMPTON30 |
|
AD, atopic dermatitis; AE, adverse event; DLQI, Dermatology Life Quality Index; EASI, Eczema Area and Severity Index
Expert presentation on real world evidence study results. Watch this video to learn about results of RELIEVE- AD:
Watch Dr. Weidinger (University Hospital Schleswig-Holstein Kiel, Germany) present 3-year results from RELIEVE-AD data.
RELIEVE-AD is a prospective, longitudinal cohort survey designed to evaluate the patient experience in the real-world setting with regard to disease control and quality of life following DUPIXENT initiation with the aim to determine potential benefits beyond those seen in the clinical trial setting.32,33
Safety
Long term safety profile
A safety and tolerability profile investigated in patients as young as 6 months old.1,4,9,14,17
Not metabolised through the liver or excreted through the kidneys1
No requirement for initial lab testing or routine lab monitoring as per the SmPC1
No known drug-drug interactions. See SmPC for live vaccines information 1*
Find out from Dr Thurein on the benefits of no monitoring with Dupixent.
The safety profile of DUPIXENT is based on a 5-year clinical trial and real-world experience in more than 750,000 patients worldwide1,8,18.
| Adult 18+4 (week 52) | Adolescent 12-1514 (week 16) | Child 6-119 (week 16) | Infants 6 months-5 years32 (week 16) | |||||
| Discontinuations due to AEs |
1.8% | 7.6% Placebo + TCS |
0.0% | 1.2% Placebo |
0.0% | 1.7% Placebo + TCS | 1.6% DUPIXENT + TCS | 1.6% Placebo + TCS |
| Rate of serious AEs |
3.6% | 5.1% Placebo + TCS |
0.0% | 1.2% Placebo |
1.7% | 1.7% Placebo + TCS | 0% DUPIXENT + TCS | 0% Placebo + TCS |
| Non-herpetic skin infections |
10.9% | 17.8% Placebo + TCS | 9.8% DUPIXENT |
18.8% |
5.8% | 13.3% Placebo + TCS | 1.6% DUPIXENT + TCS | 1.6% Placebo + TCS |
*Clinical safety and efficacy has not been established alongside live and live attenuated vaccines.
AE: adverse event; TCS: topical corticosteroids
Adverse reactions for DUPIXENT in clinical studies & post-marketing experience1
| System organ class | Frequency | Adverse reaction |
| Infection and infestations | Common | Conjunctivitis* , Oral herpes* |
| Blood and lymphatic system disorders | Common | Eosinophilia |
| Immune system disorders |
Uncommon Rare | Angioedema#, Serum sickness reactions, Serum sickness-like reactions, Anaphylactic reaction |
| Eye disorders | Common Uncommon Rare | Conjunctivitis allergic*, Keratitis*#, Blepharitis*†, Eye pruritus*†, Dry eye*†, Ulcerative keratitis*†# |
| Skin and subcutaneous tissue disorders | Uncommon | Facial rash# |
| Musculoskeletal and connective tissue disorders | Common | Arthralgia# |
| General disorders and administration site conditions | Common | Injection site reactions (includes erythema, oedema, pruritus, pain, swelling and bruising) |
|
Serious adverse reactions: eczema herpeticum, infections and immunogenicity have also been reported. | ||
- Corticosteroids
Systemic, topical, or inhaled corticosteroids should not be discontinued abruptly upon initiation of therapy with DUPIXENT. Reductions in corticosteroid dose, if appropriate, should be gradual and performed under the direct supervision of a physician.1 - Systemic Hypersensitivity
If a systemic hypersensitivity reaction (immediate or delayed) occurs, DUPIXENT should be discontinued immediately, and appropriate therapy initiated.1 - Conjunctivitis
Patients who develop conjunctivitis that does not resolve following standard treatment or signs and symptoms suggestive of keratitis should undergo ophthalmological examination, as appropriate.1 - Asthma
Patients with comorbid asthma should not adjust or stop their asthma treatments without consulting their physicians. Monitor patients with comorbid asthma carefully following discontinuation of DUPIXENT.1 - Helminth Infections
Patients with pre-existing helminth infections should be treated before initiating DUPIXENT. If patients become infected while receiving treatment with DUPIXENT and do not respond to anti-helminth treatment, DUPIXENT should be discontinued until infection resolves.1 - Vaccinations
Concurrent use of live and live attenuated vaccines with dupilumab should be avoided as clinical safety and efficacy have not been established. It is recommended that patients should be brought up to date with live and live attenuated immunisations in agreement with current immunisation guidelines prior to treatment with DUPIXENT.1
*Eye disorders and oral herpes occurred predominately in atopic dermatitis studies.
†The frequencies for eye pruritus, blepharitis, and dry eye were common and ulcerative keratitis was uncommon in atopic dermatitis studies.
# From post marketing reporting

Not metabolised through the liver or excreted through the kidneys1

No requirement for initial lab testing or routine lab monitoring as per the SmPC1

No known drug-drug interactions. See SmPC for live vaccines information 1*
Find out from Dr Thurein on the benefits of no monitoring with Dupixent.
The safety profile of DUPIXENT is based on a 5-year clinical trial and real-world experience in more than 750,000 patients worldwide1,8,18.
| Adult 18+4 (week 52) | Adolescent 12-1514 (week 16) | Child 6-119 (week 16) | Infants 6 months-5 years32 (week 16) | |||||
| Discontinuations due to AEs |
1.8% | 7.6% Placebo + TCS |
0.0% | 1.2% Placebo |
0.0% | 1.7% Placebo + TCS | 1.6% DUPIXENT + TCS | 1.6% Placebo + TCS |
| Rate of serious AEs |
3.6% | 5.1% Placebo + TCS |
0.0% | 1.2% Placebo |
1.7% | 1.7% Placebo + TCS | 0% DUPIXENT + TCS | 0% Placebo + TCS |
| Non-herpetic skin infections |
10.9% | 17.8% Placebo + TCS | 9.8% DUPIXENT |
18.8% |
5.8% | 13.3% Placebo + TCS | 1.6% DUPIXENT + TCS | 1.6% Placebo + TCS |
*Clinical safety and efficacy has not been established alongside live and live attenuated vaccines.
AE: adverse event; TCS: topical corticosteroids
Adverse reactions for DUPIXENT in clinical studies & post-marketing experience1
| System organ class | Frequency | Adverse reaction |
| Infection and infestations | Common | Conjunctivitis* , Oral herpes* |
| Blood and lymphatic system disorders | Common | Eosinophilia |
| Immune system disorders |
Uncommon Rare | Angioedema#, Serum sickness reactions, Serum sickness-like reactions, Anaphylactic reaction |
| Eye disorders | Common Uncommon Rare | Conjunctivitis allergic*, Keratitis*#, Blepharitis*†, Eye pruritus*†, Dry eye*†, Ulcerative keratitis*†# |
| Skin and subcutaneous tissue disorders | Uncommon | Facial rash# |
| Musculoskeletal and connective tissue disorders | Common | Arthralgia# |
| General disorders and administration site conditions | Common | Injection site reactions (includes erythema, oedema, pruritus, pain, swelling and bruising) |
|
Serious adverse reactions: eczema herpeticum, infections and immunogenicity have also been reported. | ||
- Corticosteroids
Systemic, topical, or inhaled corticosteroids should not be discontinued abruptly upon initiation of therapy with DUPIXENT. Reductions in corticosteroid dose, if appropriate, should be gradual and performed under the direct supervision of a physician.1 - Systemic Hypersensitivity
If a systemic hypersensitivity reaction (immediate or delayed) occurs, DUPIXENT should be discontinued immediately, and appropriate therapy initiated.1 - Conjunctivitis
Patients who develop conjunctivitis that does not resolve following standard treatment or signs and symptoms suggestive of keratitis should undergo ophthalmological examination, as appropriate.1 - Asthma
Patients with comorbid asthma should not adjust or stop their asthma treatments without consulting their physicians. Monitor patients with comorbid asthma carefully following discontinuation of DUPIXENT.1 - Helminth Infections
Patients with pre-existing helminth infections should be treated before initiating DUPIXENT. If patients become infected while receiving treatment with DUPIXENT and do not respond to anti-helminth treatment, DUPIXENT should be discontinued until infection resolves.1 - Vaccinations
Concurrent use of live and live attenuated vaccines with dupilumab should be avoided as clinical safety and efficacy have not been established. It is recommended that patients should be brought up to date with live and live attenuated immunisations in agreement with current immunisation guidelines prior to treatment with DUPIXENT.1
*Eye disorders and oral herpes occurred predominately in atopic dermatitis studies.
†The frequencies for eye pruritus, blepharitis, and dry eye were common and ulcerative keratitis was uncommon in atopic dermatitis studies.
# From post marketing reporting
Dosing
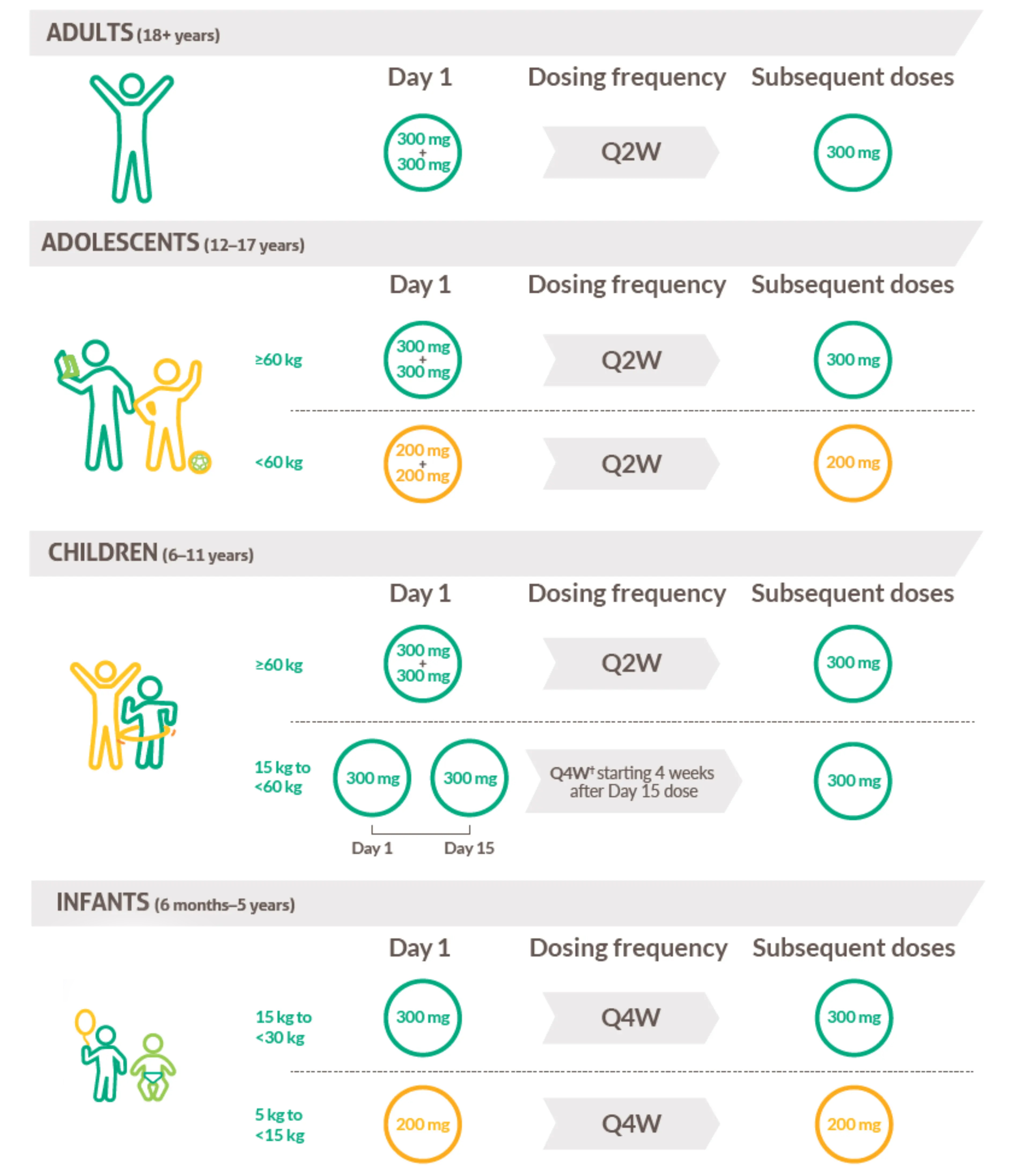
Dosing and administration1
Moderate-to-severe AD in patients aged 12+years; severe AD in patients aged 6 months-11 years.
Administration considerations in AD‡
- Rotate injection site with each injection
- Provide proper training to patients and/or caregivers on the preparations and administration of DUPIXENT prior to use, according to the Instruction for Use
- Consider completing all age-appropriate vaccinations as recommended by current immunisation guidelines prior to initiating treatment with DUPIXENT
- If an every-2-week dose is missed, instruct the patients and/or caregivers to administer the injection within 7 days from the missed dose and then resume their original schedule. If the missed dose is not administered within 7 days, instruct the patients and/or caregivers to wat util the next dose on the original schedule
- If an every-4-week dose is missed, instruct the patients and/or caregivers to administer the injection within 7 days from the missed dose and then resume their original schedule. If the missed dose is not administered within 7 days, instruct the patients and/or caregivers to administer the dose, starting a new schedule based on this date
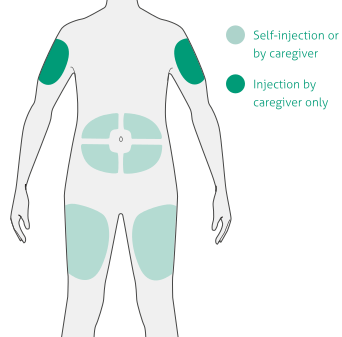
Select the injection site1:
- Stomach (except for 5cm area around navel)
- Thigh
- Outer upper arm (caregiver only)
- Do not inject into skin that is tender, damaged, bruised, or scarred
- Do not inject through clothes
- Wash hands and clean the injection site with an alcohol wipe before injecting—do not touch the injection site again or blow on it
‡Before administering DUPIXENT read complete Instructions for Use.
DUPIXENT offers 2 administration options
DUPIXENT pre-filled syringe1:
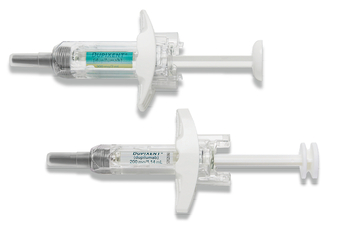
- Manual control of injection speed
- Finger grip for comfort
- Visual confirmation of injection delivery
- Needle shield
- Easy-to-carry format
- Available for use in patients aged 6 months and up by a healthcare provider or caregiver
DUPIXENT pre-filled pen1:
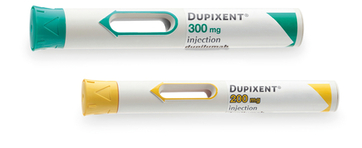
- Single-press auto-injector
- A clear, 2-step process
- Visual and audible feedback
- Hidden needle
- Compact and convenient to carry
- Minimum age restrictions apply: Please refer to the relevant PI (at the top of the page)
- Keep the pre-filled pen(s) or syringe(s) and all medicines out of the reach of children1
- Keep unused pre-filled pens or syringes in the original carton and store in the refrigerator between 2°C and 8°C1
- Do not keep pre-filled pens or syringes at room temperature (<25°C) for more than 14 days1
- Do not shake the pre-filled pen or syringe at any time1
- Do not heat the pre-filled pen or syringe1
- Do not freeze the pre-filled pen or syringe1
- Do not place the pre-filled pen or syringe into direct sunlight1
Administration1
Reimbursement
England
Dupilumab is recommended as an option for treating moderate to severe atopic dermatitis in adult patients who have not responded to at least one other systemic therapy such as ciclosporin, methotrexate, azathioprine and mycophenolate mofetil, or these are contraindicated or not tolerated.
Please click here to view the NICE Technology appraisal guidance for adult reimbursement (TA534)36
Commissioning Medicines for Children (M4C) in Specialised Services37
- NHS England will Commission treatments for patients aged less than 18 years where specific commissioning conditions within a NICE technology appraisal or NHS England policy are met in accordance with criteria outlined
- This policy applies to adolescents (12 years and older) in relation to the NICE TA534 for DUPIXENT in adults with moderate to severe AD
- This policy for adolescents aged 12 years and older is applied in NHS England specialist dermatology centres
Please follow this link for the full criteria for M4C.
Commissioning medicines for children (M4C) in specialised services37
- NH England will Commission treatments for patients aged less than 18 years where specific commissioning conditions within a nice technology appraisal or NHS England policy are met in accordance with the criteria outlined
- This policy applies to adolescents (12 years and older) with moderate to severe AD and children (6-11 years old) with severe AD in relation to the NICE technology assessment (TA534 )for DUPIXENT in adults with moderate to severe AD
- This policy for adolescents (aged 12 years and older) and children (6 to 11 years old) is applied in NHS England specialist dermatology centres
Please follow this link for the full criteria for M4C
Commissioning medicines for children (M4C) in specialised services37
- NH England will Commission treatments for patients aged less than 18 years where specific commissioning conditions within a nice technology appraisal or NHS England policy are met in accordance with the criteria outlined
- This policy applies to adolescents (12 years and older) with moderate to severe AD and children (6 months - 11 years old) with severe AD in relation to the NICE technology assessment (TA534) for DUPIXENT in adults with moderate to severe AD
- This policy for adolescents (aged 12 years and older), children (6 to 11 years old) and infants (6 months to 5 years old) is applied in NHS England specialist dermatology centres
Please follow this link for the full criteria for M4C.
Wales
All Wales medicines strategy group AWMSG advice38
- DUPIXENT is recommended as an option for use within NHS Wales in line the NICE TA534 guidance. For the full guidance please follow the link to the AWMSG advice.
All Wales medicines strategy group AWMSG advice39
- DUPIXENT is recommended as an option for use within NHS Wales. For the full guidance please follow the link to the AWMSG advice.
All Wales medicines strategy group AWMSG advice40
- DUPIXENT is recommended as an option for use within NHS Wales. For the full guidance for children please follow the link to the AWMSG advice.
Scotland
Scottish Medicines Consortium (SMC) advice41
- DUPIXENT is accepted for restricted use within NHS Scotland. For the full guidance please follow this link for guidance for SMC 2011.
Scottish Medicines Consortium (SMC) advice42
- DUPIXENT is accepted for restricted use within NHS Scotland. For the full guidance please follow this link for SMC 2232.
- Area Drug and Therapeutics Committees will be updated when paediatric license extensions are granted and highlight advice for the corresponding indication in adults; however, no SMC advice statement will be issued
Northern Ireland
In Northern Ireland DUPIXENT is accepted for use in in line with the NICE TA534 guidance.43 Follow the link to see the full guidance TA534.
In Northern Ireland DUPIXENT is accepted for use in adolescent (≥12 <18 years) in line with the SMC guidance. Follow the link to see the full guidance SMC 2232.
In Northern Ireland DUPIXENT is accepted for use in children (6-11 years) in line with the AWMSG guidance. Follow the link to see the full guidance AWMSG advice.
- DUPIXENT Summary of Product Characteristics. Great Britain. 2023
- Gandhi NA, et al. Nat Rev Drug Discov. 2016;15: 35–50.
- Guttman-Yassky E, et al. J Allergy Clin Immunol 2019; 143:155–172.
- Blauvelt A, et al. Lancet 2017; 389(10086):2287–2303
- Data on file (AD-1224 CSR EASI). Sanofi and Regeneron Pharmaceuticals, Inc. 2022
- Blauvelt A, et al. Lancet 2017; 389(10086):2287–2303. [suppl.].
- Data on file (AD-1224 CSR DLQI). Sanofi and Regeneron Pharmaceuticals, Inc. 2022
- Beck L, et al. Am J Clin Dermatol 2022; 1–16. doi: 10.1007/ s40257-022-00685-0.
- Paller AS, et al. J Am Acad Dermatol 2020; 83(5):1282–1293
- Data on file (AD-1652 CSR EASI). Sanofi and Regeneron Pharmaceuticals, Inc. 2022.
- Data on file (AD-1652 CSR pruritus NRS). Sanofi and Regeneron Pharmaceuticals, Inc. 2022
- Data on file (AD-1652 CSR CDLQI). Sanofi and Regeneron Pharmaceuticals, Inc. 2022
- Simpson EL, et al. Dupilumab Efficacy and Safety in Adolescents With Moderate-to-Severe Atopic Dermatitis: Results From a Multicenter, Randomized, Placebo-Controlled, Double-Blind, Parallel Group, Phase 3 Study (Poster PA-17) presented at the 43rd annual Hawaii Dermatology Seminar;Waikoloa, HI, USA, February 17–22 2019
- Simpson EL, et al. JAMA Dermatol 2020; 156(1):44-56
- Data on file (AD-1526 EASI). Sanofi and Regeneron Pharmaceuticals, Inc. 2022
- Data on file (AD-1526 CDLQI). Sanofi and Regeneron Pharmaceuticals, Inc. 2022.
- Paller AS, et al. Presented at the Revolutionizing Atopic Dermatitis (RAD)Virtual Conference; December 11, 2022
- Data on File (patient numbers). Sanofi and Regeneron Pharmaceuticals, Inc. 2023
- De Bruin-Weller M, et al. Presented at the 27th Annual European Academy of Dermatology and Venereology Conference (EADV 2018); Sep 12-16, 2018; Paris, France.
- Paller AS, et al. Dupilumab for Adolescents With Moderate-to-Severe Atopic Dermatitis: Results From a Phase 3, Randomized, Double-Blinded Trial (Poster 10051) presented at the 77th annual meeting of the American Academy of Dermatology, Washington, DC, USA, March 1–5 201
- Gittler JK, et al. J Allergy Clin Immunol. 2012; 130(6):1344-1354.
- Paller AS, et al. Lancet. 2022;400(10356):908–919
- Simpson EL, et al. J Am Acad Dermatol. Published online February 23, 2024. https://doi.org/10.1016/j.jaad.2023.12.066.
- Simpson EL, et al. J Am Acad Dermatol. Published online February 23, 2024. https://doi.org/10.1016/j.jaad.2023.12.066. [suppl.]
- Sanofi Data on File. SAGB.DUP.17.09.1168. September 2017
- Simpson EL, et al. New Engl J Med. 2016;375(24):2335–2348
- Bajwa D, et al. Real-world response to dupilumab in patients with atopic dermatitis: a retrospective review of 100 patients. Poster P81. Presented at the 101st British Association of Dermatologists Annual meeting, Virtual, July 6–8 2021
- Kreeshan FC, et al. Dermatol Ther (Heidelb). 2021;11(1):149–160.
- Sears AV, et al. Br J Dermatol. 2021;184(4):755–757..
- O’Driscoll D, et al. Dupilumab, an incoming treatment for adolescents with atopic dermatitis: experience from a tertiary eczema. Abstract PA01. Presented at the 99th British Association of Dermatologists Annual meeting, Liverpool, July 2–4 2019.
- Gardette E, et al. Realworld experience of dupilumab for the treatment of severe recalcitrant atopic dermatitis in a tertiary centre. Abstract O06. Presented at the 99th British Association of Dermatologists Annual meeting, Liverpool, July 2–4 2019
- Strober B, Mallya UG, Yang M, et al. Treatment Outcomes Associated With DUPIXENT Use in Patients With Atopic Dermatitis: 1-Year Results From the RELIEVE-AD Study. JAMA Dermatol. 2022;158(2):142–150. doi:10.1001/jamadermatol.2021.4778
- Strober B, et al. DUPILUMAB IMPROVES PATIENT-REPORTED OUTCOMES AMONG ADULTS WITH MODERATE-TO-SEVERE ATOPIC DERMATITIS (AD) IN CLINICAL PRACTICE: 30- TO 36-MONTH RESULTS FROM THE RELIEVE-AD STUDY [conference presentation]. AAD 2022 Annual Meeting Boston, Massachusetts. March 25-29, 2022
- Data on file (AD-1539 Safety). Sanofi and Regeneron Pharmaceuticals, Inc 2022
- DUPIXENT Summary of Product Characteristics. Ireland. 2024
- NICE [2022] Dupilumab for treating moderate to severe atopic dermatitis. Available at: https://www.nice.org.uk/guidance/ta534/chapter/1-Recommendations. Date last accessed: March 2025. All rights reserved. Subject to notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/ publication.
- NHS England [2017] Commissioning medicines for children in specialised services (170001/P). Available at https://www.england.nhs.uk/publication/commissioning-medicines-for-children-specialised-services/ Date last accessed: March 2025.
- All Wales Medicines Strategy Groups. Dupilumab (DUPIXENT). Available at: https://awttc.nhs.wales/accessing-medicines/medicine-recommendations/dupilumab-dupixent2/ Date last accessed: March 2025.
- All Wales Medicines Strategy Groups. Dupilumab (DUPIXENT). Available at: https://awttc.nhs.wales/accessing-medicines/medicine-recommendations/dupilumab-dupixent/ Date last accessed: March 2025.
- All Wales Medicines Strategy Groups. Dupilumab (DUPIXENT). Available at: https://awttc.nhs.wales/accessing-medicines/medicine-recommendations/dupilumab-dupixent5/ Date last accessed: March 2025.
- Scottish Medicines Consortium. Dupilumab (DUPIXENT). Available at: https://www.scottishmedicines.org.uk/medicines-advice/dupilumab-dupixent-fullsubmission-smc2011//. Date last accessed: March 2025.
- Scottish Medicines Consortium. Dupilumab (DUPIXENT). Available at: https://www.scottishmedicines.org.uk/medicines-advice/dupilumab-dupixent-abbreviated-smc2232/. Date last accessed: March 2025.
- Northern Ireland Formulary Dupilumab (DUPIXENT). Available at: https://niformulary.hscni.net/managed-entry/managed-entry-decision/. Date last accessed: March 2025.
MAT-XU-2304409 (v4.0) Date of Preparation March 2025