- Article
- Source: Campus Sanofi
- 7 Jan 2026
Unseen Burdens: How Cumulative Life Course Impairment (CLCI) Affects Patients with Moderate-to-Severe Atopic Dermatitis (AD)
DUPIXENT® (dupilumab) Prescribing Information UK
Introduction
AD is a chronic inflammatory skin condition characterised by intense itching and periodic flares of inflamed, dry skin which may eventually impair patients' quality of life (QoL).1,2 The underlying pathophysiology of AD typically involves type 2 inflammation (T2I), driven by cytokines such as IL-4 and IL-13, which exacerbate symptoms and contribute to the disease's complexity.3 However, the burden of uncontrolled moderate-to-severe AD can extend far beyond the visible symptoms, encompassing psychological and social impact that can contribute to CLCI.2,4
According to the Global Burden of Disease study, AD has the highest disability-adjusted life-year (DALY) burden among all skin diseases, with an age-standardised DALY rate significantly higher than psoriasis and urticaria.2
This article focuses on understanding CLCI in AD, and the importance of recognising and addressing these hidden burdens.
Moderate-to-Severe Atopic Dermatitis–A Cumulative Burden
The multidimensional burdens of AD in patients with moderate-to-severe symptoms include more than just skin lesions and itching.1,5,6 Patients frequently suffer from specific elements of CLCI such as stigma, psychological distress, limited social support, and coping challenges, all of which contribute to CLCI.4
Stigma
Patients with AD often face social stigma due to overt public rejection, poor self-image, and lack of self-confidence4
Psychological Burden
Psychological comorbidities of people living with AD can include anxiety, depression and suicidal ideation — further complicating the patient's health profile4
Systemic Implications
The chronic inflammation associated with AD can lead to comorbidities and AD is associated with conditions such as poor bone health, cardiovascular diseases, hypertension, stroke, Type 2 diabetes, and other immune-related conditions7
Social Support
Additionally, the lack of adequate social support from family, friends, and colleagues can hinder effective management of the disease4
Coping Challenges
Maladaptive coping, denial, helplessness and family factors add to coping challenges. These challenges add to the cumulative burden, making it difficult for patients to maintain a normal lifestyle4
The cumulative effect of these burdens over the years can lead to a potentially irreversible damage, affecting various life domains such as productivity at school and work, and the ability to maintain social relationships.2,4,8
Listen to Dr Hywel Cooper discuss AD and the impact on patients QoL
CLCI–Potentially Irreversible Damage Due to Inadequate Treatment and Uncontrolled AD2,4,7,9,10
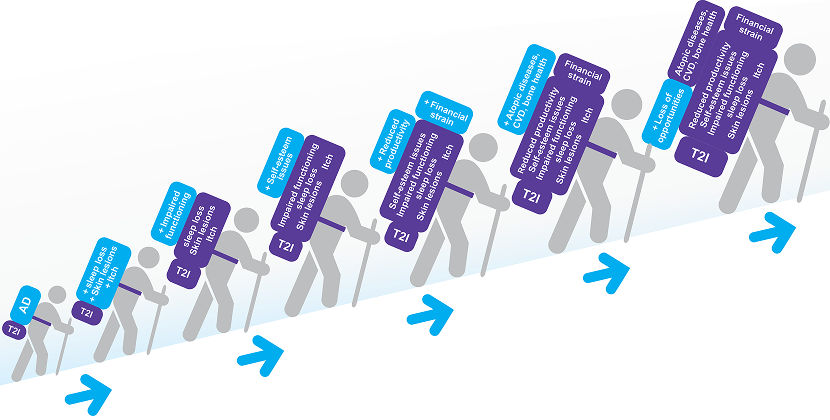
Timely identification and treatment escalation of patients with uncontrolled AD may improve the signs, symptoms and quality of life burdens these patients are currently experiencing.8
Conclusion
The burdens of uncontrolled AD may become cumulative, impacting various aspects of a patient's life and contributing to CLCI.1,4,6 By understanding and addressing these multifaceted challenges, healthcare professionals can improve patient outcomes and quality of life.2,8
Early identification of at-risk patients, using appropriate tools, and timely escalation to appropriate treatments are crucial in providing best practice care for patients.4,8,11
Discover tools that can help you identify AD patients that are uncontrolled.
DUPIXENT is approved for use in eligible patients as young as 6 months old in AD.12
DUPIXENT is indicated for use in patients aged 12 years and older with moderate-to-severe atopic dermatitis (AD), and in patients aged 6 months to 11 years of age with severe AD, who are candidates for systemic therapy.12
AD, atopic dermatitis; CLCI, Cumulative Life Course Impairment; DALY, disability-adjusted life-year; IL, interleukin; QoL, quality of life; T2I, type 2 inflammation.
References
- Weidinger S et al. Lancet. 2016; 387(10023): 1109—1122.
- Fasseeh AN et al. Dermatol Ther (Heidelb). 2022; 12(12): 2653—2668.
- Moniaga CS et al. Diagnostics (Basel). 2021; 11(11): 2090.
- Augustin M, et al. JEADV. 2022; 36(7): 3—16.
- Weidinger S et al. Br J Dermatol. 2024; 190(6): 846—857.
- Calzavara-Pinton P et al. Adv Ther. 2023; 40(12): 5366—5382.
- Thyssen JP et al. J Allergy Clin Immunol. 2023; 151(5): 1155—1162.
- von Stülpnagel CC et al. J Eur Acad Dermatol Venereol. 2021; 35(11): 2166—2184.
- Davis DMR et al. J Am Acad Dermatol. 2022; 86(6): 1335—1336.e18.
- Bacci ED et al. J Dermatolog Treat. 2023; 34(1): 2202288.
- Bieber T. Nat Rev Drug Discov. 2023; 22(8): 662—680.
- DUPIXENT Summary of Product Characteristics. 2025.

Atopic Dermatitis Control Test (ADCT)
Atopic dermatitis, a type of eczema, may be affecting your patient’s life in more ways than you know.
The ADCT gives a measure of how controlled your patient’s eczema is. Use these 6 concise questions to evaluate all dimensions of atopic dermatitis control.
Development of ADCT involved literature review as well as interviews with patients and physicians, and was funded by Sanofi and Regeneron.
Try the ADCTMAT-XU-2504918 (v1.0) Date of Preparation: January 2026