- Article
- Source: Campus Sanofi
- 3 Dec 2025
The Hidden Impact of Cumulative Life Course Impairment (CLCI) in Dermatology: An Overview
DUPIXENT® (dupilumab) Prescribing Information UK
Introduction
Skin diseases are the fourth most common cause of nonfatal morbidity, impacting nearly one-third of the global population. Chronic diseases cause substantial burden, and despite their high prevalence, this burden is often underestimated.1,2
Chronic skin diseases including atopic dermatitis (AD), notably affect patients' health-related quality of life (HRQoL), including physical health, emotional well-being, and social interactions. Patients with inflammatory skin diseases often suffer from symptoms that contribute to psychological distress, including anxiety and depression, ultimately resulting in cumulative long-term impairment.3 Understanding CLCI is essential to fully grasp the enduring burden of chronic skin diseases and the irreversible impact on patients' lives over time.4
CLCI in Dermatology: What You Need to Know?
CLCI is a theoretical concept that describes the non-reversible burden of chronic skin diseases over time. In some cases, this persistent burden can lead to chronic impairment and missed opportunities resulting in lasting psychosocial and personal damage. Some individuals are less vulnerable to CLCI, while others are more affected by factors such as stigma, physical comorbidities, and psychological comorbidities. Difficulty in coping with these factors increases the patients' risk of vulnerability.4
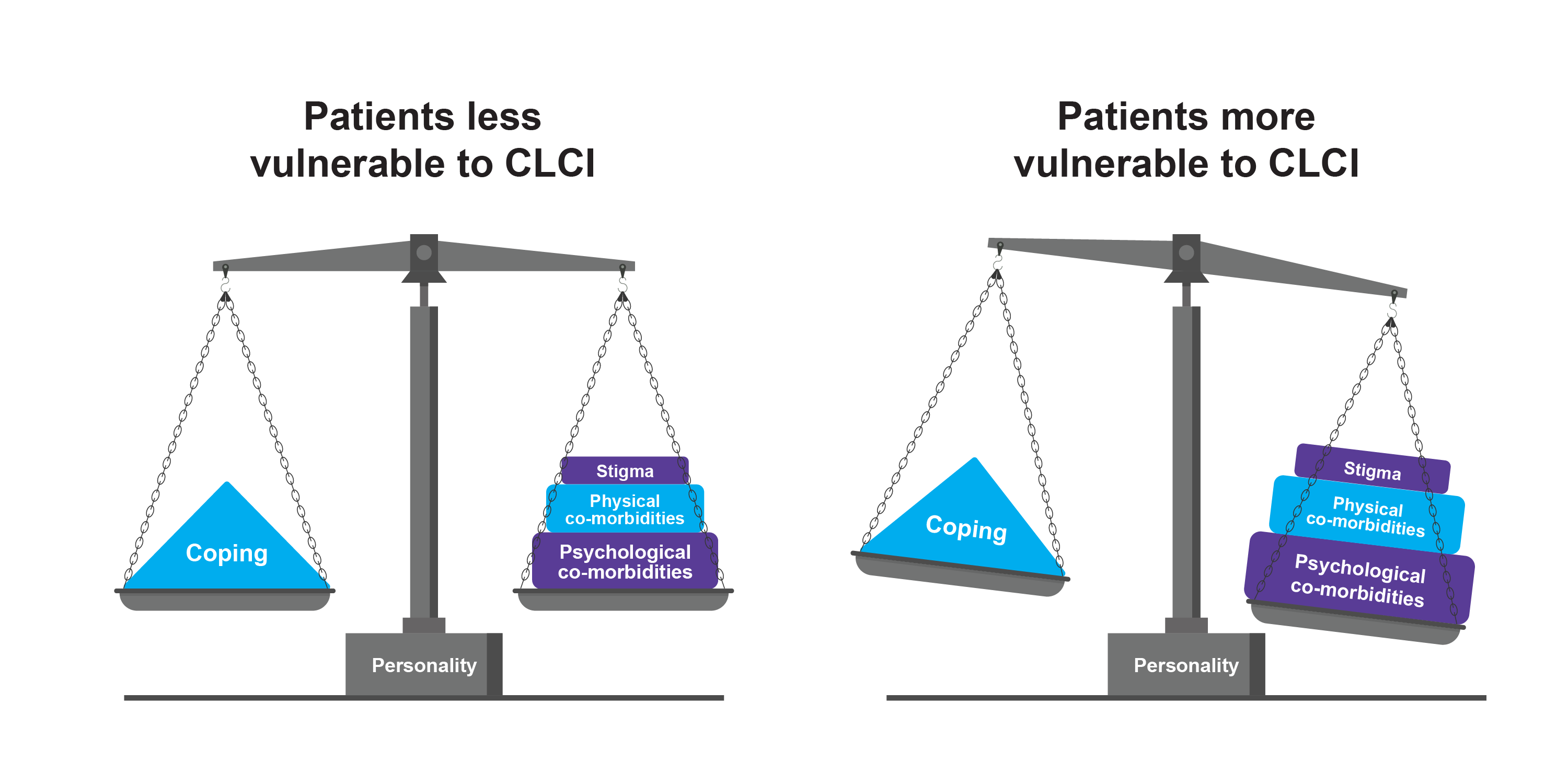
Hypothetical impairment over a patient’s life course4
Adapted from Augustin A et al. 2013.4
Recognising these factors is crucial for healthcare professionals to develop comprehensive management strategies aimed at mitigating the long-term effects of chronic skin diseases such as AD.
The Chronic Nature of AD: A Multidimensional Burden
AD is a chronic, relapsing condition marked by eczematous lesions and severe pruritus. Although AD often manifests in infancy, affecting approximately 20% of children, it remains highly prevalent in adults, indicating a lifelong disposition.5
The chronic nature of AD can lead to a continuous cycle of flare-ups and remissions. The atopic comorbidities such as food allergies, asthma, allergic rhinitis, other immune-mediated inflammatory diseases, and non-atopic comorbidities such as mental health disorders, may develop in patients with severe disease.4–6
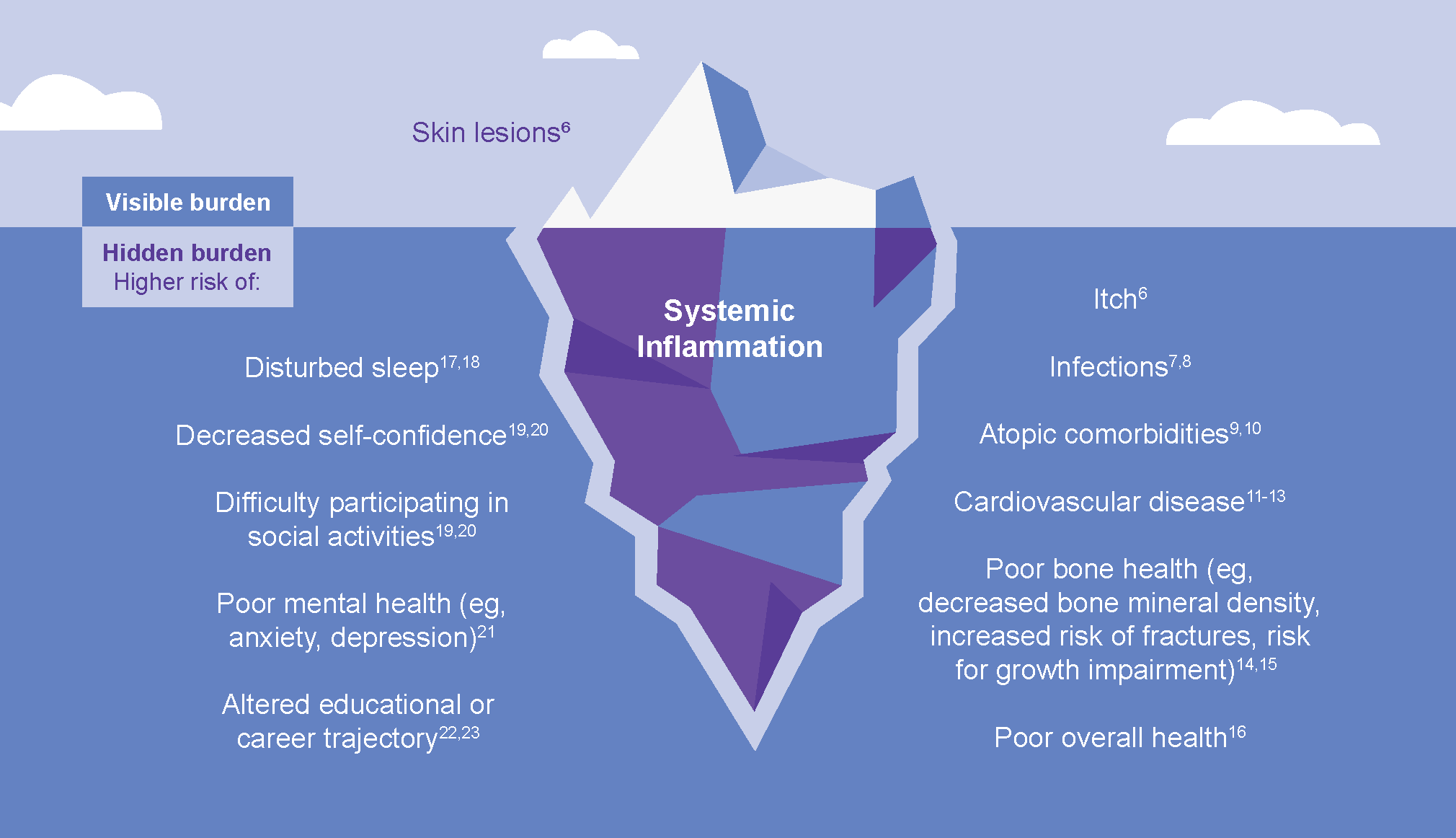
The burden of uncontrolled AD may further lead to other factors such as sleep loss, impaired functioning, self-esteem issues, reduced productivity, cardiovascular diseases, poor bone health, financial strain, and loss of opportunities.23–27
Listen to Dr Antonia Lloyd-Lavery discuss the comorbidities associated with AD
High Disease Burden in Low BSA Involvement
This chronic and relapsing nature of AD underscores the importance of evaluating the disease's impact, even when the Body Surface Area (BSA) involvement is minimal.
Patients with a low BSA involvement may experience a high disease burden.28 The severity of symptoms and their impact on QoL are not solely dependent on the extent of skin involvement but also on the functionality and visibility of the affected areas.28,29
Hands and feet and face are commonly reported to be affected by AD. These areas can lead to significant physical impairments that affect the QoL, causing psychological distress and disrupting work and school productivity.29–34
Over time, these ongoing challenges can contribute to CLCI, highlighting the long-term, cumulative burden of chronic skin diseases.3 Timely intervention and escalation of therapy for patients with uncontrolled moderate to severe AD can be critical in helping to manage the signs, symptoms and quality of life impacts of uncontrolled AD.35
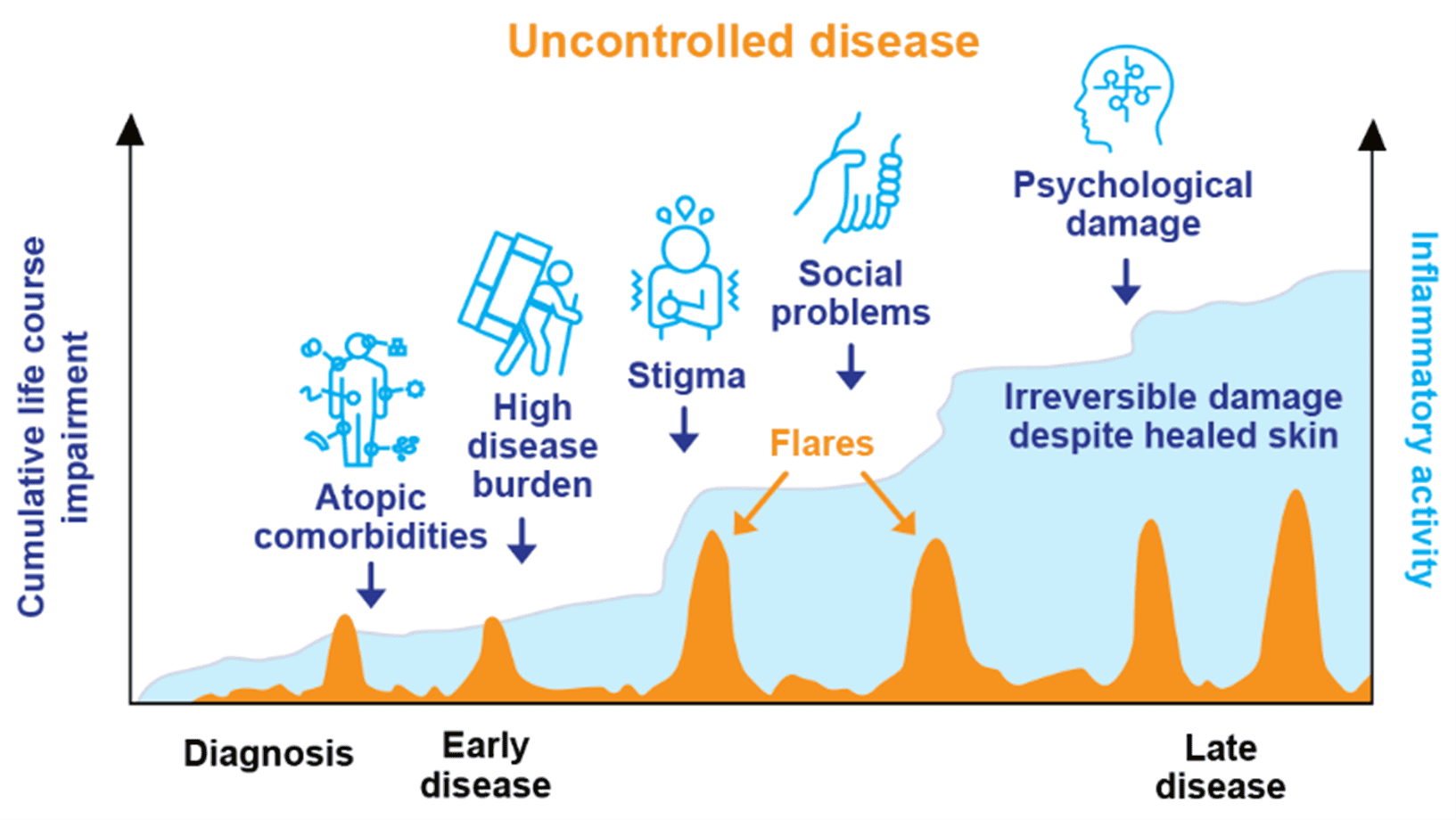
The graph is a hypothetical model of CLCI throughout the course of AD, adapted from Kimball, et al. J Eur Acad Dermatol Venereol. 201027 and Bieber T, et al. Nat Rev Drug Discov. 2023.36
Conclusion
CLCI is a crucial concept in understanding the long-term impact of chronic skin conditions like AD.3,4 For patients with moderate-to-severe AD, the burden extends beyond the skin, causing psychological and social distress, and affecting the overall QoL.29–31
Timely and appropriate escalation for patients suffering with uncontrolled AD may help reduce the burdens they face.35
Watch Prof Michael Cork discuss disease modification in AD
DUPIXENT is approved for use in eligible patients as young as 6 months old in AD.37
DUPIXENT is indicated for use in patients aged 12 years and older with moderate-to-severe atopic dermatitis (AD), and in patients aged 6 months to 11 years of age with severe AD, who are candidates for systemic therapy.37
References
- Seth D et al. Curr Dermatol Rep. 2017; 6(3): 204–210.
- Flohr C et al. Br J Dermatol. 2021;184: 189–190.
- von Stülpnagel CC et al. J Eur Acad Dermatol Venereol. 2021; 35(11):2166–2184.
- Augustin A et al. Wound Medicine. 2013; 1: 2–6.
- Weidinger S et al. Lancet. 2016; 387(10023): 1109–1122.
- Simpson EL et al. J Am Acad Dermatol. 2016; 74(3): 491–498.
- Wang V et al. Ann Allergy Asthma Immunol. 2021;126(1): 3–12.
- Cameron S et al. Allergy. 2024; 79(1):26–36.
- Weidinger S et al. Br J Dermatol. 2024; 190(6): 846–857.
- Calzavara-Pinton P et al. Adv Ther. 2023; 40(12): 5366–5382.
- Lee SW et al. Allergy Asthma Immunol Res. 2023;15(2): 231–245.
- Lundin S et al. J Eur Acad Dermatol Venereol. 2023; 37(9): 1854–1862.
- Wan J et al. J Allergy Clin Immunol Pract. 2023; 11(10):3123–3132.e3.
- Silverberg JI et al. JAMA Dermatol. 2015; 151(4): 401–409.
- Wu D et al. Ann Transl Med. 2021; 9(1): 40.
- Silverberg JI et al. J Invest Dermatol. 2015; 135: 56–66.
- Chang YS et al. J Allergy Clin Immunol. 2018; 142(4): 1033–1040.
- Paller AS et al. J Am Acad Dermatol. 2022; 87(5): 1104–1108.
- Paller AS et al. Dermatol Ther (Heidelb). 2023; 13(4): 961–980.
- Neri I et al. J Asthma Allergy. 2023; 16: 383–396.
- Girolomoni G et al. Dermatol Ther (Heidelb). 2021; 11(1): 117–130.
- Ibler K et al. Dermatol Reports. 2011; 3(1): e5.
- Bacci ED et al. J Dermatolog Treat. 2023; 34(1): 2202288.
- Fasseeh AN et al. Dermatol Ther (Heidelb). 2022; 12(12): 2653–2668.
- Davis DMR et al. J Am Acad Dermatol. 2022; 86(6): 1335–1336.e18.
- Thyssen JP et al. J Allergy Clin Immunol. 2023; 151(5): 1155–1162.
- Kimball AB et al. J Eur Acad Dermatol Venereol. 2010; 24(9):989–1004.
- Silverberg JI et al. Dermatitis. 2023; 34(2): 135–144.
- Simpson EL et al. J Am Acad Dermatol. 2024; 90(6): 1190–1199.
- Grant L et al. Adv Ther. 2020; 37(2): 692–706.
- Silverberg JI et al. J Am Acad Dermatol. 2023; 89(3): 519–528.
- Jaros J et al. Dermatitis. 2020; 31(3): 169–177.
- Rønnstad ATM et al. J Am Acad Dermatol. 2024; 90: 616–618.
- Moore RA et al. Rule of Nines. Available at: https://www.ncbi.nlm.nih.gov/books/NBK513287/. Date last accessed: December 2025.
- Davis DMR et al. J Am Acad Dermatol. 2024; 90(2): e43-e56.
- Bieber T. Nat Rev Drug Discov. 2023;22(8):662-680.
- DUPIXENT Summary of Product Characteristics. 2025.

Atopic Dermatitis Control Test (ADCT)
Atopic dermatitis, a type of eczema, may be affecting your patient’s life in more ways than you know.
The ADCT gives a measure of how controlled your patient’s eczema is. Use these 6 concise questions to evaluate all dimensions of atopic dermatitis control.
Development of ADCT involved literature review as well as interviews with patients and physicians, and was funded by Sanofi and Regeneron.
Try the ADCTMAT-GB-2500201 (v1.0) Date of Preparation: December 2025