- Resource
- Source: Campus Sanofi
- 6 Oct 2025
Formulary & Service Support

This section has resources that you may require for your formulary submissions and business cases or policy reviews. As well as product information, therapy support resources and treatment guidelines. You can download these materials to better support your decision making.
Dermatology

Dupixent® (dupilumab) Formulary Application Support Pack
This document provides factual and technical information about Dupixent to support you with relevant document for local formulary applications.
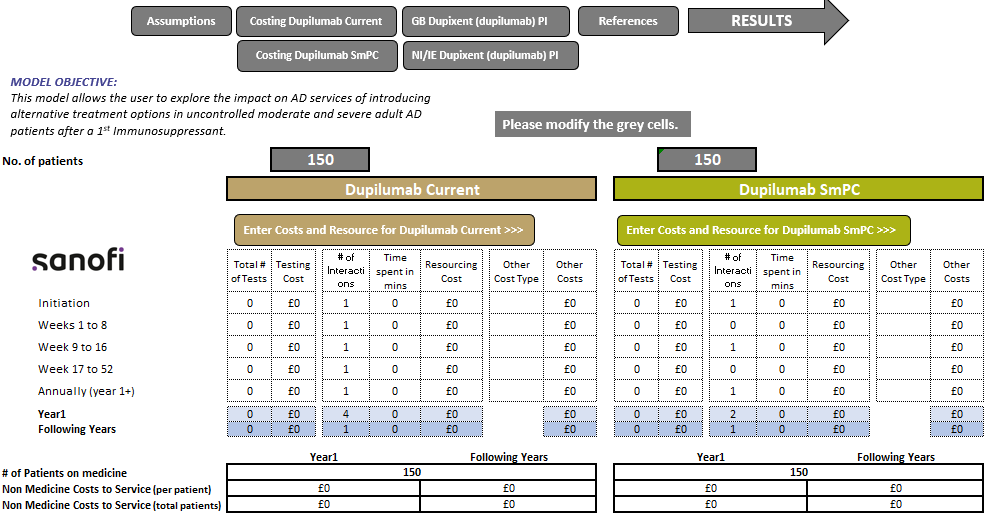
Dupixent® (dupilumab) Optimisation Model
The impact on resource utilisation when using Dupixent in accordance with the SmPC.
Diabetes

Tzield®▼ (teplizumab) Value Proposition Summary
Understand the value and impact Tzield can bring to your population.

Tzield®▼ (teplizumab) Information Guide
This document provides factual and technical information about TZIELD to support your understanding of the product and relevant documentation for local applications.
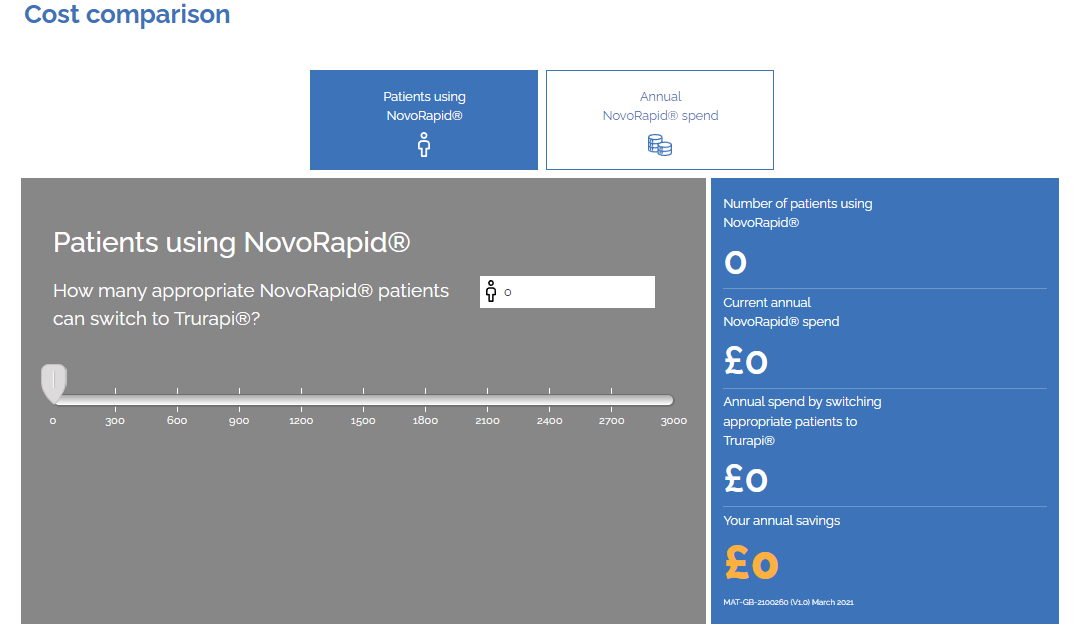
Trurapi®▼ (insulin aspart 100 units/mL)
Simulate your savings from switching to Trurapi.
Respiratory

COPD - Eligible Population Estimator
This document provides information on estimating patients who might be eligable for Dupixent® via the COPD indication.
COPD - Burden Analysis Model
Explore the impact that biologics could have on COPD services in regard to costs, admissions and stays within hospital.
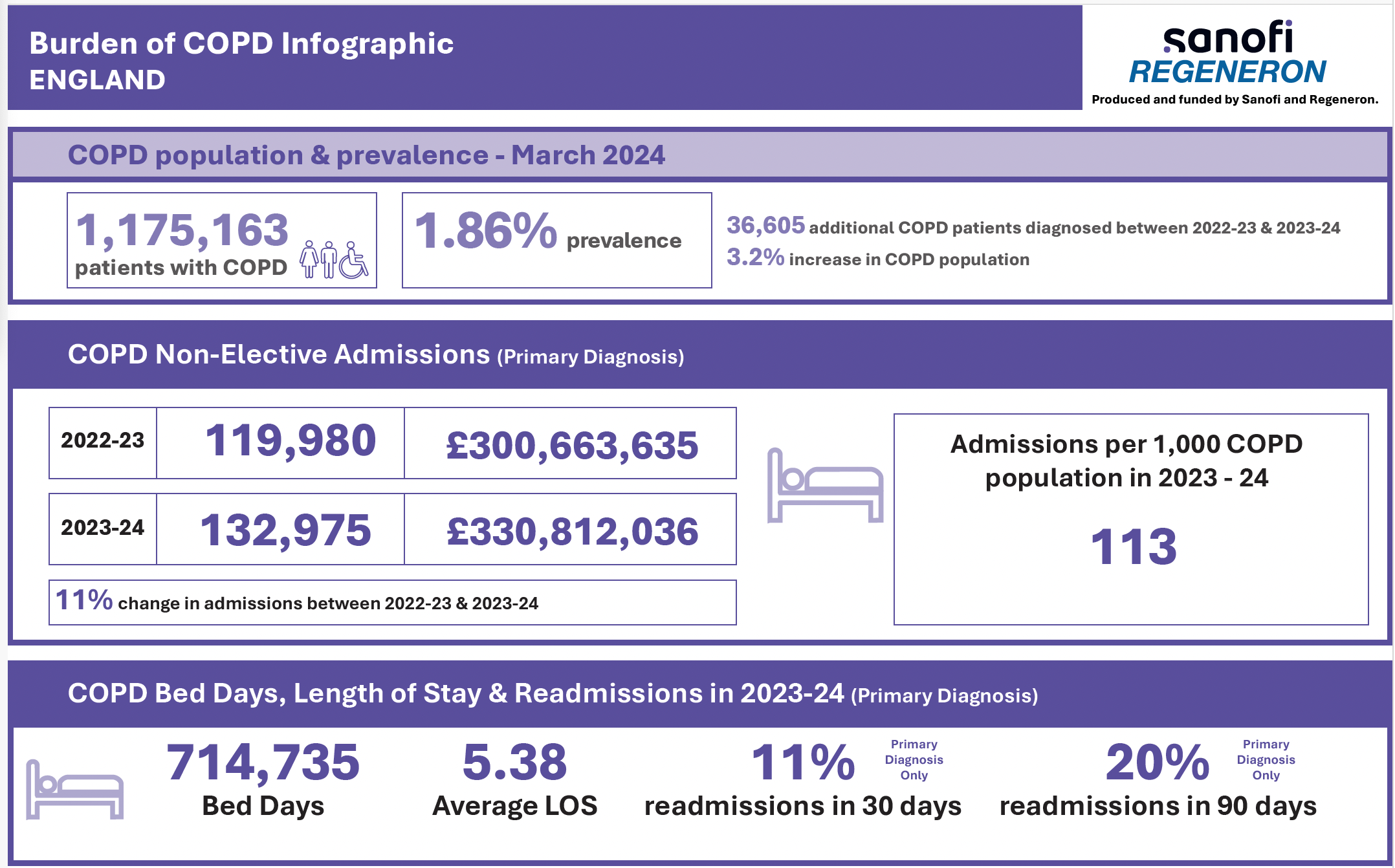
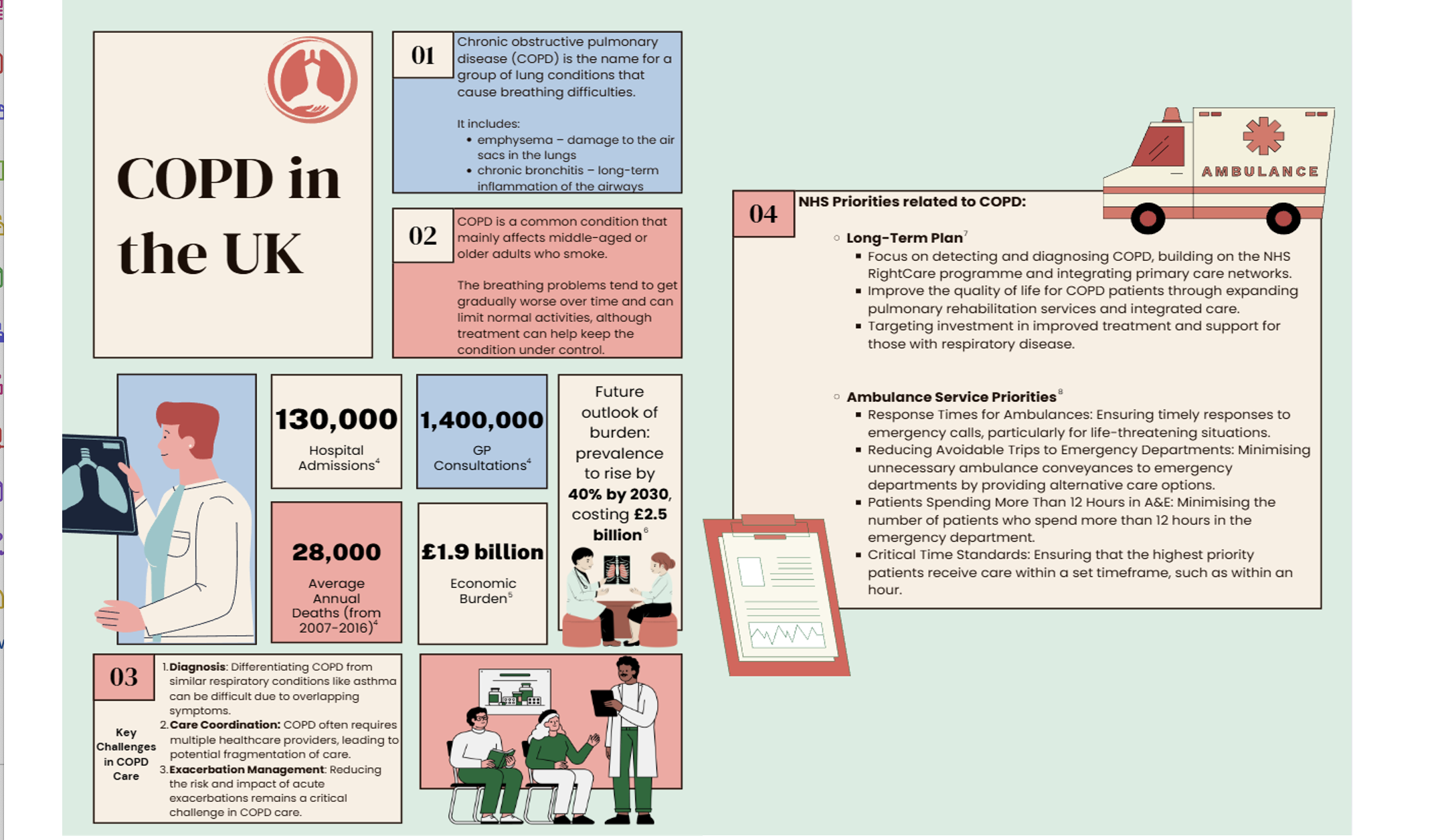
Burden of COPD Infographic
An infographic showing the burden of COPD on hospital admissions, bed days and length of stay in England. For data relating to your ICB, please Get in Touch via the link below.

.jpg)
MAT-XU-2401933 (v3.0) Date of Preparation: November 2025