INDICATION: TZIELD is indicated to delay the onset of Stage 3 T1D in adult and paediatric patients 8 years of age and older with Stage 2 T1D.1
TZIELD (teplizumab) is the first and only disease-modifying therapy that delays the natural progression of T1D through preservation of beta cell function1-3
Traditionally, autoimmune T1D treatment has focused on symptomatic control through the replacement of endogenous insulin with insulin replacement therapy. TZIELD is the first proactive intervention available to help address the immunological root cause of autoimmune T1D.1,4-7
Disease-modifying therapy for Stage 2 T1D
TZIELD is a humanised anti-cluster of differentiation 3 (CD3) monoclonal antibody designed to target and modulate the autoimmune process involved in beta cell destruction*1,6
Preservation of pancreatic beta cells
TZIELD helps to preserve beta cell function and insulin production in the pancreas, thereby delaying progression from presymptomatic Stage 2 to symptomatic Stage 3 autoimmune T1D1,8
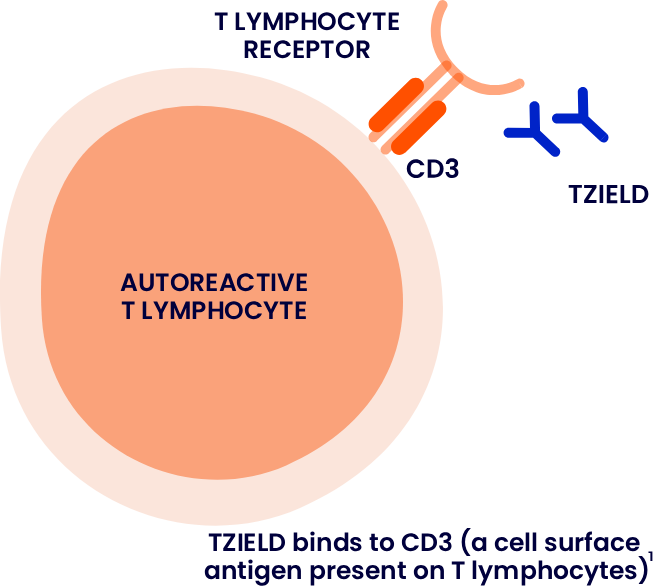
*The mechanism of action for TZIELD is not fully understood. It may involve partial agonistic signalling and deactivation of pancreatic beta cell autoreactive T lymphocytes.1
Get in Touch with Us
Questions? Leave your details and we'll reach out to you at your preferred time.
Get in touchCD3, cluster of differentiation 3; T1D, Type 1 diabetes.
- TZIELD® (teplizumab) UK Summary of Product Characteristics. 2025.
- Kuhn C and Weiner HL. Immunotherapy. 2016; 8(8): 889–906.
- Herold KC, et al. Diabetes Care. 2023; 46(10): 1848–1856.
- Subramanian S, et al. BMJ. 2024; 384: e075681.
- Nagy G, et al. World J Diabetes. 2022; 13(10): 835–850.
- Ramos EL, et al. N Engl J Med. 2023; 389(23): 2151–2161.
- Herold KC, et al. Nat Rev Immunol. 2024; 24(6): 435–451.
- Sims EK, et al. Sci Transl Med. 2021;13 (583): eabc8980.
MAT-XU-2500765 (v1.0) | November 2025
