
Cerdelga is indicated for the long-term treatment of adult patients with Gaucher disease type 1 (GD1), who are CYP2D6 poor metabolisers (PMs), intermediate metabolisers (IMs) or extensive metabolisers (EMs).
Paediatric population (from 6 to <18 years of age) weighing ≥25 kg
Cerdelga is indicated for paediatric patients with GD1 who are 6 years and older with a minimum body weight of 25 kg, who are stable on enzyme replacement therapy (ERT), and who are CYP2D6 PMs, IMs or EMs.1
Profile
Confidence in Cerdelga®▼ (eliglustat) - A first-line oral therapy for adults with Gaucher disease type 1 with long-term efficacy data in treatment-naïve patients and patients previously stabilised on enzyme replacement therapy.1
.jpg)
Cerdelga® demonstrated comparable efficacy to enzyme replacement therapy (ERT) in patients previously stabilised on ERT through maintenance of patient stability across haematological and visceral parameters1
In the clinical trials, the proportion of patients who confirmed preference for an oral treatment was 94% (93/99)2
Cerdelga® demonstrated comparable efficacy to enzyme replacement therapy (ERT) in patients previously stabilised on ERT through maintenance of patient stability across haematological and visceral parameters1
In the clinical trials, the proportion of patients who confirmed preference for an oral treatment was 94% (93/99)2
Evidence
Efficacy
Pivotal study of eliglustat in treatment-naïve GD1 patients – study 02507 (ENGAGE)
Study 02507 was a randomized, double-blind, placebo-controlled, multicentre clinical study in 40 patients with GD1. In the eliglustat group 3 (15%) patients received a starting dose of 42 mg eliglustat twice daily during the 9-month primary analysis period and 17 (85%) patients received a dose escalation to 84 mg twice daily based on plasma trough concentration.
Cerdelga® demonstrated efficacy vs placebo in visceral (spleen and liver) and haematological parameters.1
In treatment-naïve patients, Cerdelga® demonstrated efficacy vs placebo in visceral and haematologic parameters over 9 months, with continued improvements in Cerdelga®-treated patients for up to 4.5 years.1*
*All patients transitioned to treatment after 9 months.
Pivotal study of eliglustat in GD1 patients switching from ERT - Study 02607 (ENCORE)
The ENCORE study was a randomised, open-label, active-controlled, non-inferiority, multicentre clinical study in 159 patients previously stabilised with ERT.3
As part of the ENCORE study Cerdelga® demonstrated comparable efficacy to imiglucerase in maintenance of patients’ stability across visceral and haematologic parameters in patients previously stabilized on ERT.**1 The stability in visceral and haematologic parameters (haemoglobin levels, platelet count, and spleen and liver volume) was maintained for up to 4 years.†1
ENCORE study: Percentage of patients meeting the primary composite endpoint of all four components after 12 months of treatment.
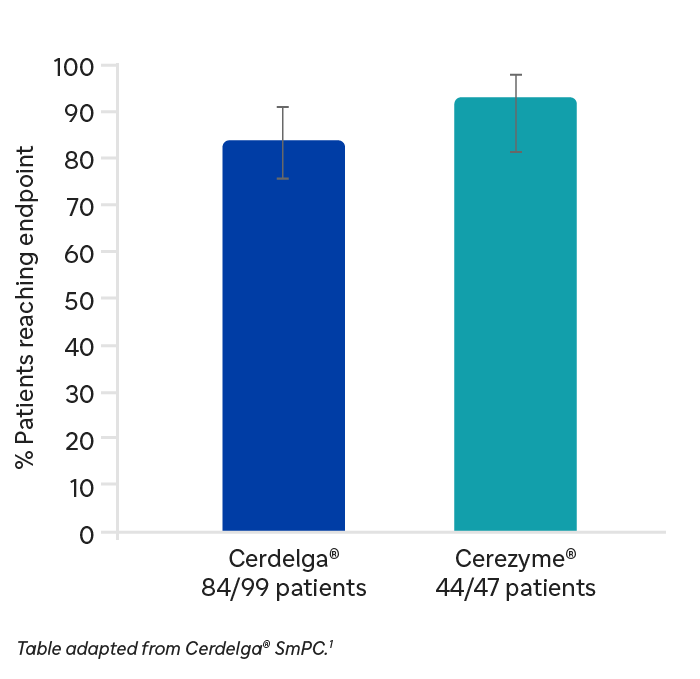
**Excludes patients with a total splenectomy and all patients transitioned to Cerdelga® after 52 weeks.
†Excludes patients with a total splenectomy.
Long-term clinical outcomes in treatment-naive GD1 patients - Study 304
In an open-label, single-arm, multicentre, phase 2 study in 26 patients, Cerdelga® showed sustained improvements in organ volume and haematologic parameters over an 8-year treatment period in treatment-naive patients (n=18).‡1
‡18 patients completed 8 years of treatment. 16 patients had an efficacy endpoint assessment at year 8.
Cerdelga® improved skeletal measures in treatment-naïve patients and patients previously stabilised on ERT1
In Study 02507 (ENGAGE), during CERDELGA treatment in treatment-naive patients previously stabilised on ERT, skeletal measures (bone marrow burden [BMB] and bone mineral density [BMD]) decreased by a mean of 1.1 points in eliglustat treated patients (n=19) compared to no change in patients receiving placebo (n=20).1
In study 304, the phase 2, open-label, single arm, multicenter study of eliglustat in 26 patients, Cerdelga® improved BMD from osteopenia to normal range after 4 years.3
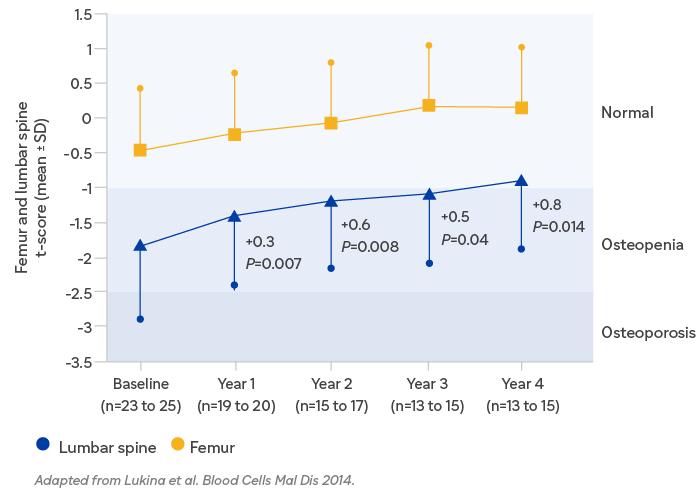
In study 304, the phase 2, open-label, single arm, multicenter study of eliglustat in 26 patients, Cerdelga® improved BMD from osteopenia to normal range after 4 years.3
Long-term clinical outcomes in treatment-naive GD1 patients - Study 304
In an open-label, single-arm, multicentre, phase 2 study in 26 patients, Cerdelga® showed sustained improvements in organ volume and haematologic parameters over an 8-year treatment period in treatment-naive patients (n=18).‡1
‡18 patients completed 8 years of treatment. 16 patients had an efficacy endpoint assessment at year 8.
Cerdelga® improved skeletal measures in treatment-naïve patients and patients previously stabilised on ERT1
In Study 02507 (ENGAGE), during CERDELGA treatment in treatment-naive patients previously stabilised on ERT, skeletal measures (bone marrow burden [BMB] and bone mineral density [BMD]) decreased by a mean of 1.1 points in eliglustat treated patients (n=19) compared to no change in patients receiving placebo (n=20).1
In study 304, the phase 2, open-label, single arm, multicenter study of eliglustat in 26 patients, Cerdelga® improved BMD from osteopenia to normal range after 4 years.3

In study 304, the phase 2, open-label, single arm, multicenter study of eliglustat in 26 patients, Cerdelga® improved BMD from osteopenia to normal range after 4 years.3
Safety profile
Cerdelga® is generally of manageable tolerability1
- The most commonly reported AE with Cerdelga® is dyspepsia, in approximately 6% of the pooled adult clinical trial patients.1
| Adverse reactions1 System organ class | Common (≥1/100 to <1/10) |
|---|---|
| Nervous System disorders | Headache*, dizziness*, dysgeusia |
| Cardiac disorders | Palpitations |
Respiratory, thoracic and mediastinal disorders | Cough, Throat irritation |
Gastrointestinal disorders | Dyspepsia, abdominal pain upper*, diarrhoea*, nausea, constipation, abdominal pain*, gastroesophageal reflux disease, abdominal distension*, gastritis, dysphagia, vomiting*, dry mouth, flatulence |
| Skin and subcutaneous tissue disorders | Dry skin, urticaria* |
| Musculoskeletal and connective tissue disorders | Arthralgia, pain in extremity*, back pain* |
| General disorders and administration site conditions | Fatigue |
*The incidence of the adverse reaction was the same or higher with placebo than with Cerdelga in the placebo-controlled pivotal study.
The overall adverse reaction profile of Cerdelga® is based on a total of 393 patients from four studies between the ages of 16-75 years who received eliglustat for a mean duration of 3.6 years (up to 9.3 years). This includes the pooled results from the primary analysis periods and extension periods of two pivotal Phase 3 studies (ENGAGE and ENCORE), one 8-year, long term Phase 2 study (Study 304) and one supporting Phase 3b study (EDGE).4
Mechanism of Action
Cerdelga® is a potent and specific inhibitor of glucosylceramide synthase. Cerdelga® is a substrate reduction therapy (SRT) with clinical studies showing wide distribution to tissues, including bone marrow.1 Cerdelga® reduces the rate of synthesis of the major substrate glucosylceramide (GL-1) to match its impaired rate of catabolism in patients with Gaucher disease Type 1.1 Cerdelga® is a ceramide-based analogue (versus amino-sugar-based analogue). It is a specific inhibitor of glucosylceramide synthase85–7 and does not inhibit intestinal glucosidases.5,6
Usage
CYP2D6 testing
Cerdelga® should only be used in patients who, based on genotyping, have a predicted CYP2D6 poor, intermediate or extensive metaboliser phenotype. Determination of the patient's CYP2D6 phenotype is required prior to initiating treatment with Cerdelga®.1
Genotyping to determine the patient's CYP2D6 phenotype is to be performed using an established genetic laboratory test that is able to detect a specific set of CYP2D6 alleles with adequate accuracy, sensitivity and specificity in order to ensure consistent identification of CYP2D6 metaboliser status.5
Sanofi offers a CYP2D6 testing service to UK clinicians treating patients with Gaucher disease through Archimed Life. However, there are several suitable commercial tests available.
In the UK, for more information on gaining access to testing via Archimed Life, or for accredited laboratories, you can contact Sanofi Medical Information on 0800 035 2525, or your local representative.
References
- Cerdelga®. Summary of Product Characteristics.
- Cox TM et al. Lancet. 2015 Jun 13;385(9985):2355–62.
- Lukina E et al. Blood Cells Mol Dis. 2014 Dec;53(4):274–6.
- Peterschmitt et al. Orphanet Journal of Rare Diseases. (2019). 14:128.
- Belmatoug N et al. Eur J Intern Med. 2016. pii: S0953–6205(16)30217–5.
- McEachern KA et al. Mol Genet Metab. 2007;91:259–267
- Shayman JA. Drugs Fut. 2010;35:613–620.
MAT-XU-2204064 (v7.0) Date of preparation: February 2026