
30 years supporting HCPs in the VTE management
Clexane is in stock in the UK across all doses and we are confident in our ability to supply. If you are experiencing issues with stock, please contact Customer service on 0800 854 430.
Are you a prescriber?
Resources
Patient Supporting Materials

Summary of Clexane support materials

Switching to Clexane?
Patient Supporting Materials

Summary of Clexane support materials

Switching to Clexane?
The prophylaxis of venous thromboembolism (VTE):1
- In moderate and high risk surgical patients, in particular those undergoing orthopaedic or general surgery including cancer surgery.
- In medical patients with an acute illness and reduced mobility at increased risk of VTE.
The treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), excluding PE likely to require thrombolytic therapy or surgery:1
- Extended treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) and prevention of its recurrence in patients with active cancer.
- Prevention of thrombus formation in extracorporeal circulation during haemodialysis.
- Acute coronary syndrome:
- Treatment of unstable angina and Non ST-segment elevation myocardial infarction (NSTEMI), in combination with oral acetylsalicylic acid.
- Treatment of acute ST-segment elevation myocardial infarction (STEMI) including patients to be managed medically or with subsequent percutaneous coronary intervention (PCI).
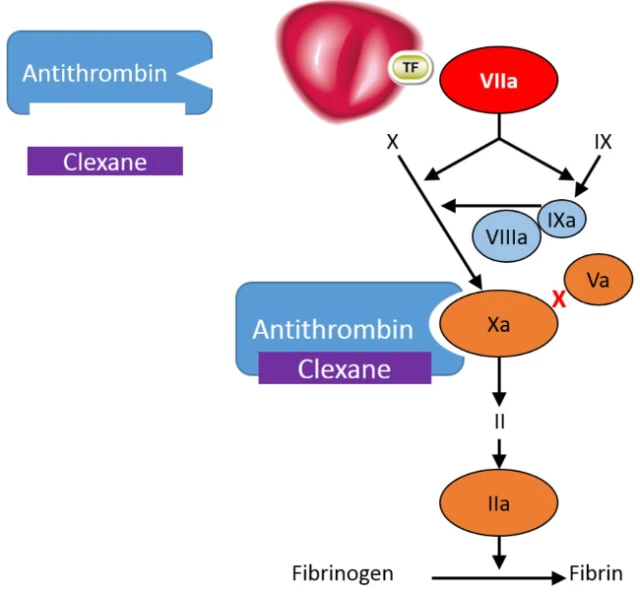
Clexane® exerts its anticoagulant effect by preventing the formation of blood clots through binding to antithrombin, a naturally occurring coagulation inhibitor and potentiating its action.2
Antithrombin is a natural inhibitor of the coagulation factors FXIa, FIXa, FXa and FIIa (thrombin).2
Clexane® forms a complex with antithrombin. This complex undergoes a conformational change; in its altered conformation, the complex inhibits FXa, which is the primary mechanism of action. It also inhibits FIIa, although to a lesser extent - in vitro, Clexane has a high anti-Xa activity and low anti-IIa or anti thrombin activity, with a ratio of 3.6 :1.2
.png)
Clexane is indicated in adults for the extended treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) and prevention of its recurrence in patients with active cancer based on the RIETECAT trial results.
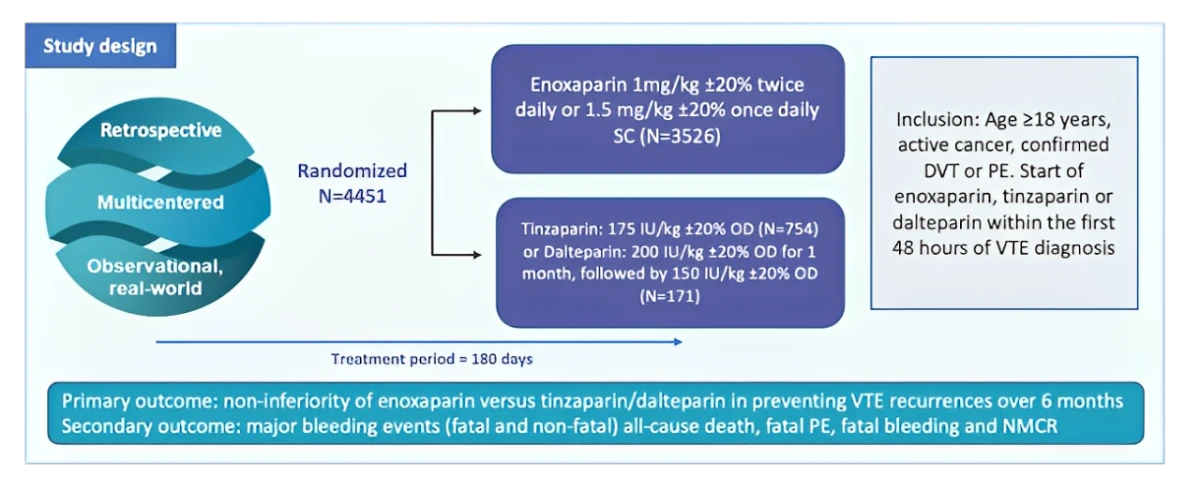
RIETECAT trial is a multinational observational retrospective cohort study using data from the RIETE registry.3
It is the first and largest study comparing the effectiveness and safety of enoxaparin against dalterparin and tinzaparin in a real-world cohort of cancer patients with VTE.3
.png)
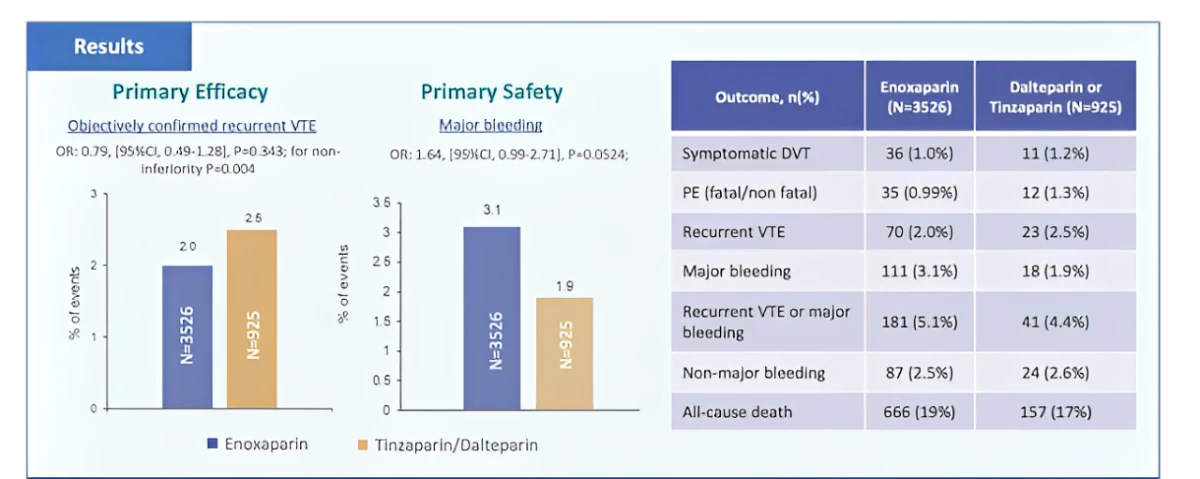
Cancer patients with VTE receiving full dose enoxaparin or tinzaparin/dalteparin had comparable effectiveness and safety outcomes over a 6 month period.
OR: 0.79 (95%CI. 049-1.28) p=0.004
The efficacy outcome was a composite of symptomatic DVT and fatal or non-fatal PE and the safety outcome was a composite of major bleeding events (fatal or non fatal) and non major bleeds of clinical significance.
Due to the non-interventional nature of the study, comparability of patients between groups may be difficult to achieve; however multivariate Cox model was used to minimize the confounding bias.
Patients treated with enoxaparin had a non-significantly lower risk for VTE recurrences, a non-significantly higher risk for major bleeding, and similar risks for non-major bleeding or death over 6 months compared to tinzaparin/dalteparin.
The prophylaxis of venous thromboembolism (VTE):1
- In moderate and high risk surgical patients, in particular those undergoing orthopaedic or general surgery including cancer surgery.
- In medical patients with an acute illness and reduced mobility at increased risk of VTE.
The treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), excluding PE likely to require thrombolytic therapy or surgery:1
- Extended treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) and prevention of its recurrence in patients with active cancer.
- Prevention of thrombus formation in extracorporeal circulation during haemodialysis.
- Acute coronary syndrome:
- Treatment of unstable angina and Non ST-segment elevation myocardial infarction (NSTEMI), in combination with oral acetylsalicylic acid.
- Treatment of acute ST-segment elevation myocardial infarction (STEMI) including patients to be managed medically or with subsequent percutaneous coronary intervention (PCI).

Clexane® exerts its anticoagulant effect by preventing the formation of blood clots through binding to antithrombin, a naturally occurring coagulation inhibitor and potentiating its action.2
Antithrombin is a natural inhibitor of the coagulation factors FXIa, FIXa, FXa and FIIa (thrombin).2
Clexane® forms a complex with antithrombin. This complex undergoes a conformational change; in its altered conformation, the complex inhibits FXa, which is the primary mechanism of action. It also inhibits FIIa, although to a lesser extent - in vitro, Clexane has a high anti-Xa activity and low anti-IIa or anti thrombin activity, with a ratio of 3.6 :1.2
.png)
Clexane is indicated in adults for the extended treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) and prevention of its recurrence in patients with active cancer based on the RIETECAT trial results.
RIETECAT trial is a multinational observational retrospective cohort study using data from the RIETE registry.3
It is the first and largest study comparing the effectiveness and safety of enoxaparin against dalterparin and tinzaparin in a real-world cohort of cancer patients with VTE.3
.png)
Cancer patients with VTE receiving full dose enoxaparin or tinzaparin/dalteparin had comparable effectiveness and safety outcomes over a 6 month period.
OR: 0.79 (95%CI. 049-1.28) p=0.004
The efficacy outcome was a composite of symptomatic DVT and fatal or non-fatal PE and the safety outcome was a composite of major bleeding events (fatal or non fatal) and non major bleeds of clinical significance.
Due to the non-interventional nature of the study, comparability of patients between groups may be difficult to achieve; however multivariate Cox model was used to minimize the confounding bias.
Patients treated with enoxaparin had a non-significantly lower risk for VTE recurrences, a non-significantly higher risk for major bleeding, and similar risks for non-major bleeding or death over 6 months compared to tinzaparin/dalteparin.
.jpg)
Footnote
CAT=cancer-associated thrombosis; VTE=Venous Thromboembolism; CI=confidence interval; OR=Odds Ratio; SC=subcutaneous; IU=international unit; OD=Once daily; PE pulmonary embolism; NMCR=Non-major clinically relevant.
References
- Clexane Pre-filled Syringes Summary of Product Characteristics. February 2022. Please consult the SmPC for full information.
- Carter NJ, McCormack PL, Plosker GL (2008). Enoxaparin: a review of its use in ST-segment elevation myocardial infarction. Drugs, 68:691–710.
- Trujillo-Sanos J et al. Res Pract Thromb Haemost. 2022;6:e12736.
MAT-XU-2204397 (v6.0) Date of Preparation: August 2025
