
Profile
Quadrivalent Influenza Vaccine▼ (Split Virion, Inactivated) High-Dose (QIV-HD) is indicated for the active immunisation against infection caused by influenza viruses in adults 60 years of age and older.1 The use of Quadrivalent Influenza Vaccine (Split Virion, Inactivated) High‐Dose should be in accordance with official recommendations on vaccination against influenza.
An amendment to the National flu immunisation guidance for 2024-25 for both England and Wales has been issued to include Quadrivalent Influenza Vaccine (Split Virion, Inactivated) High-Dose (QIV-HD) as a first line recommended reimbursable option for the over 65 year old cohort. In addition, QIV-HD will be a first line recommended reimbursable option for the 60-64 year olds in the at risk cohort for the 2024/2025 influenza season.6,7
QIV-HD is available in a 10-pack, with no attached needles (separate needles are provided by Sanofi free of charge for the 2024-25 influenza season).
Before you prescribe or administer this vaccine please refer to the SmPC.
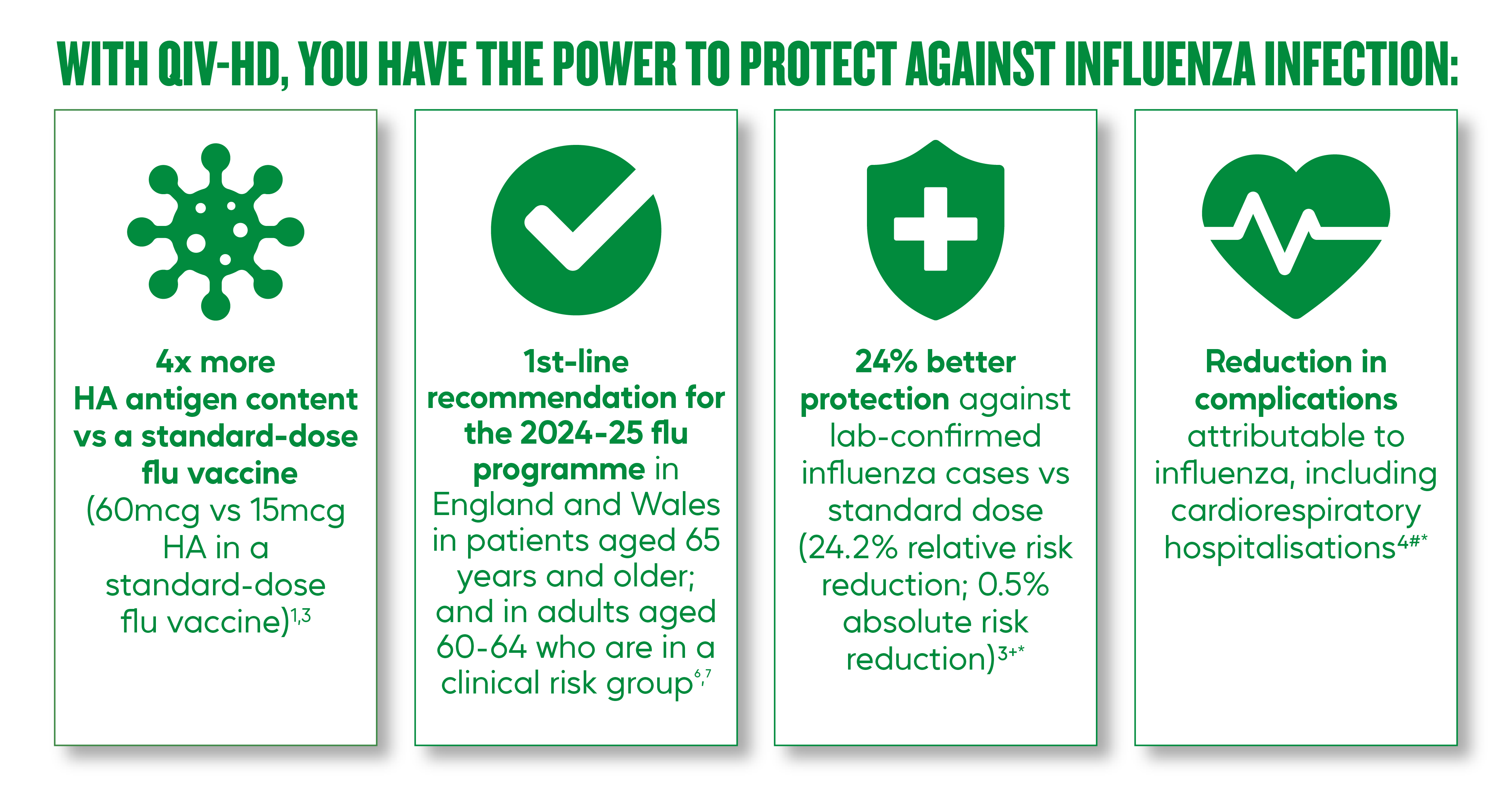
How effective is QIV-HD?
Our commitment is to ensure patients have access to influenza vaccines that help protect them from what really matters: hospitalisations due to flu infection or attributable to flu.
With QIV-HD you have the power to protect:
- Protect against influenza protection
- Help protect beyond flu by preventing flu related hospitalisations.1,3,4
High dose has been shown to be 24.2% (9.7-36.5%) greater relative risk reduction (0.5% absolute risk reduction) than Standard Dose at preventing lab confirmed influenza infections, in adults aged 65 years and older*3.
A meta-analysis published in 2023 including 12 publications (6 RCT and 15 observational studies) of studies conducted over 12 seasons (multiple circulating strains) found QIV-HD* was:
- 27.8% (12.5-40.5) more effective than SD in preventing hospitalisations due to pneumonia.4
- 16.7% (13.8-19.5) more effective than SD in preventing cardiorespiratory hospitalisations.4
Who can have QIV-HD?
QIV-HD is licensed for use in the UK for adults aged 60 and over1. Guidance within the National Flu Immunisation Program 2024 to 2025 Letters for both England and Wales provides a first line recommendation for QIV-HD to be used in the following cohorts: patients aged 65 years and older; and in adults aged 60-64 who are in a clinical risk group.6,7
What is the safety profile of QIV-HD?
In clinical trials the safety profile of HD has been shown to be comparable to that of SD vaccines1,5
QIV-HD is generally well-tolerated. The most frequently reported adverse reaction after vaccination with QIV-HD was injection-site pain, reported by 42.6% of study participants, followed by myalgia (23.8%), headache (17.3%), and malaise (15.6%).1 Please refer to the QIV-HD SmPC for full list of adverse events.
How can I order QIV-HD?
QIV-HD can be ordered online now for delivery later in the year as part of the upcoming 2024-25 season.
Please visit www.vaxishop.co.uk to place an order
Usage of QIV-HD1
| Indication | Quadrivalent Influenza Vaccine (Split Virion, Inactivated) High-Dose is indicated for active immunisation in adults 60 years of age and older for the prevention of influenza disease. The use of Quadrivalent Influenza Vaccine (Split Virion, Inactivated) High-Dose should be based in accordance with official recommendations on vaccination against influenza. |
|---|---|
| Presentation | 0.7 ml of suspension in pre-filled syringe (Type I glass) equipped with a plunger stopper (bromobutyl rubber) and a tip-cap. Pack of 10 pre-filled syringes without needles. Quadrivalent Influenza Vaccine (Split Virion, Inactivated) High-Dose, after shaking gently, is a colourless opalescent liquid. |
| Posology | In adults 60 years of age and older: one dose of 0.7 ml. The safety and effectiveness of Quadrivalent Influenza Vaccine (Split Virion, Inactivated) High-Dose in children less than 18 years of age have not been established. |
| Administration | The preferred route of administration for this vaccine is intramuscular although it may also be given subcutaneously. The recommended site for intramuscular injection is the deltoid region. The vaccine should not be injected into the gluteal region, or into areas where there may be a major nerve trunk. |
| Further doses | Annual influenza vaccination is recommended because immunity during the year after vaccination declines and because circulating strains of influenza virus change from year to year. |
| Age range | Adults 60 years of age and over. |
*This study used Trivalent Influenza Vaccine (Split Virion, Inactivated) High Dose. Other studies have shown that the efficacy and effectiveness results of TIV-HD are inferred to QIV-HD given the demonstration of statistically comparable immunogenicity between TIV-HD and QIV-HD.1
QIV-HD is generally well-tolerated. The most frequently reported adverse reaction after vaccination with QIV-HD was injection-site pain, reported by 42.6% of study participants, followed by myalgia (23.8%), headache (17.3%), and malaise (15.6%).1 Please refer to the QIV-HD SmPC for full list of adverse events.
+In a randomised controlled trial (RCT) of 31,803 subjects, HD-TIV showed 24.2% (95% CI: 9.7 to 36.5) better protection against lab-confirmed influenza cases of any circulating strain vs standard-dose influenza vaccine1,3
#HD-TIV pooled relative vaccine efficacy/effectiveness results: Over 12 seasons in >45 million adults 65+ showed that HD-IIV provided better protection than SD-IIV against influenza-like illness and influenza-related hospitalisations, as well as cardiovascular, cardiorespiratory, and all-cause hospitalisations4. 11% fewer influenza hospitalisation 11.2%; 95% CI: 7.4 to 14.8); 17% fewer cardiorespiratory hospitalisations (16.7%; 95% CI: 13.8 to 19.5); 28% fewer pneumonia hospitalisations (27.8%; 95% CI: 12.5 to 40.5); 8% fewer all-cause hospitalisations (8.2%; 95% CI: 5.5 to 10.8).
QIV-HD is indicated for active immunisation in adults 60 years of age and older for the prevention of influenza disease. The use of QIV-HD should be based in accordance with official recommendations on vaccination against influenza.1
References
- Quadrivalent Influenza Vaccine High Dose (QIV-HD) Summary of Product Characteristics
- JCVI Statement on Influenza Vaccines 2024-25. Available at: https://app.box.com/s/t5ockz9bb6xw6t2mrrzb144njplimfo0/file/1289995245447 Accessed: June 2024
- DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371(1):635–45
- Lee JKH, Lam GKL, Yin JK, Loiacono MM, Samson SI. High-dose influenza vaccine in older adults by age and seasonal characteristics: Systematic review and meta-analysis update. Vaccine X. 2023 Jun 5;14:100327. doi: 10.1016/j.jvacx.2023.100327. PMID: 37333054; PMCID: PMC10276206
- Chang LJ, Meng Y, Janosczyk H, Landolfi V, Talbot HK; QHD00013 Study Group. Safety and immunogenicity of high dose quadrivalent influenza vaccine in adults ≥65 years of age: A phase 3 randomized clinical trial. Vaccine. 2019 Sep 16;37(39):5825-5834. doi: 10.1016/j.vaccine.2019.08.016. Epub 2019 Aug 17. PMID: 31431411
- National flu immunisation programme 2024 to 2025 letter for England. Available at: (https://www.gov.uk/government/publications/national-flu-immunisation-programme-plan-2024-to-2025/statement-of-amendment-to-the-annual-flu-letter-for-2024-to-2025-12-june-2024) Accessed June 2024
- NHS Wales Flu Letter 2024-2025. Available at: (https://www.gov.wales/influenza-vaccines-and-eligible-cohorts-2024-2025-season-whc2023047-html) Accessed June 2024
MAT-XU-2402066 (v1.0) Date of preparation: June 2024