
IMROZ: The first positive phase 3 trial of frontline anti-CD38 + VRd therapy in patients with NDMM who are ineligible for ASCT.1
For adult patients with transplant-ineligible newly diagnosed multiple myeloma, improve outcome with SARCLISA® in combination with bortezomib, lenalidomide and dexamethasone (VRd) vs. VRd.*1,2
*SARCLISA + VRd demonstrated superior PFS vs. VRd alone (63.2% of patients’ progression free at 5-year follow up vs 45.2% with VRd alone, HR 0.06 [98.5% CI 0.41, 0.88]; p<0.01).1
Indication
SARCLISA® is indicated, in combination with bortezomib, lenalidomide, and dexamethasone, for the treatment of adult patients with newly diagnosed multiple myeloma who are ineligible for autologous stem cell transplant (ASCT)2
Efficacy
SARCLISA + VRd (IMROZ): Trial design
IMROZ: The first positive phase 3 trial of frontline anti-CD38 + VRd therapy in patients with NDMM who are ineligible for ASCT.1
A large, randomised, open-label, multicentre, phase 3 trial that compared SARCLISA + VRd to VRd alone in newly diagnosed patients with multiple myeloma who are ineligible for transplant.1
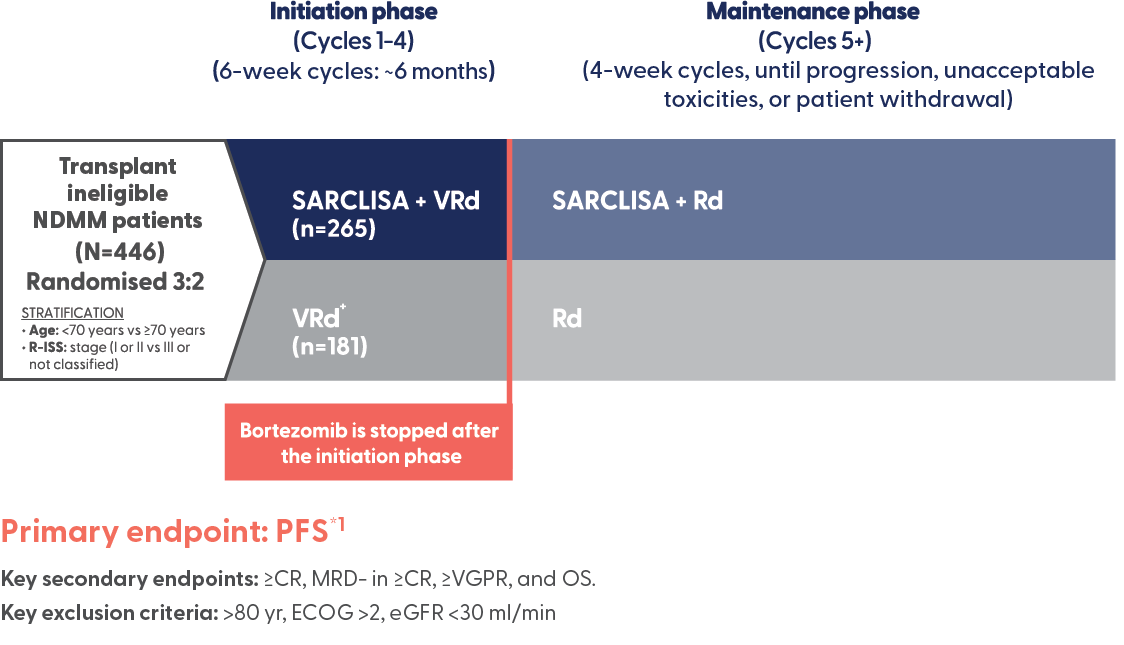
*The interim analysis of PFS was performed after 162 events of disease progression or death had occurred (73% of the planned 222 events for the final analysis).1
†In the maintenance phase, patients randomised to the VRd arm who experienced progressive disease during the Rd treatment period could cross over to receive SARCLISA + Rd.1
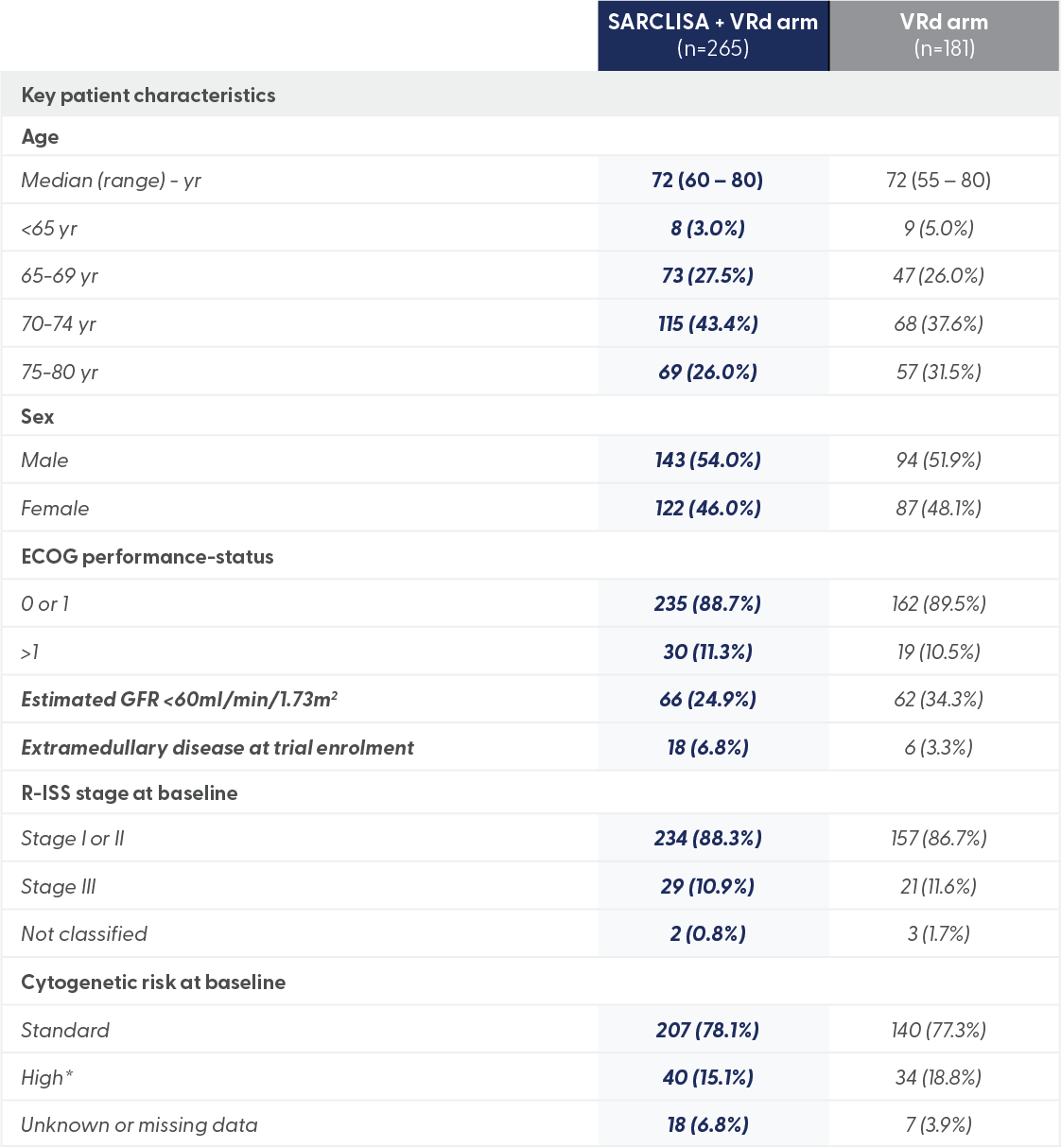
*High cytogenetic risk was defined as the presence of del(17p), t(4;14), t(14;16), or a combination of these.
A large, randomised, open-label, multicentre, phase 3 trial that compared SARCLISA + VRd to VRd alone in newly diagnosed patients with multiple myeloma who are ineligible for transplant.1

*The interim analysis of PFS was performed after 162 events of disease progression or death had occurred (73% of the planned 222 events for the final analysis).1
†In the maintenance phase, patients randomised to the VRd arm who experienced progressive disease during the Rd treatment period could cross over to receive SARCLISA + Rd.1

*High cytogenetic risk was defined as the presence of del(17p), t(4;14), t(14;16), or a combination of these.
SARCLISA + VRd (IMROZ): Efficacy
63.2% of patients remain alive and progression-free at a median follow-up of 60 months with SACLISA + VRd vs 45.5% for VRd.1,2,3-5
SARCLISA + VRd mPFS: not reached at 59.7 months vs 54.3 months in the VRd arm (95% CI 45.2-NR).
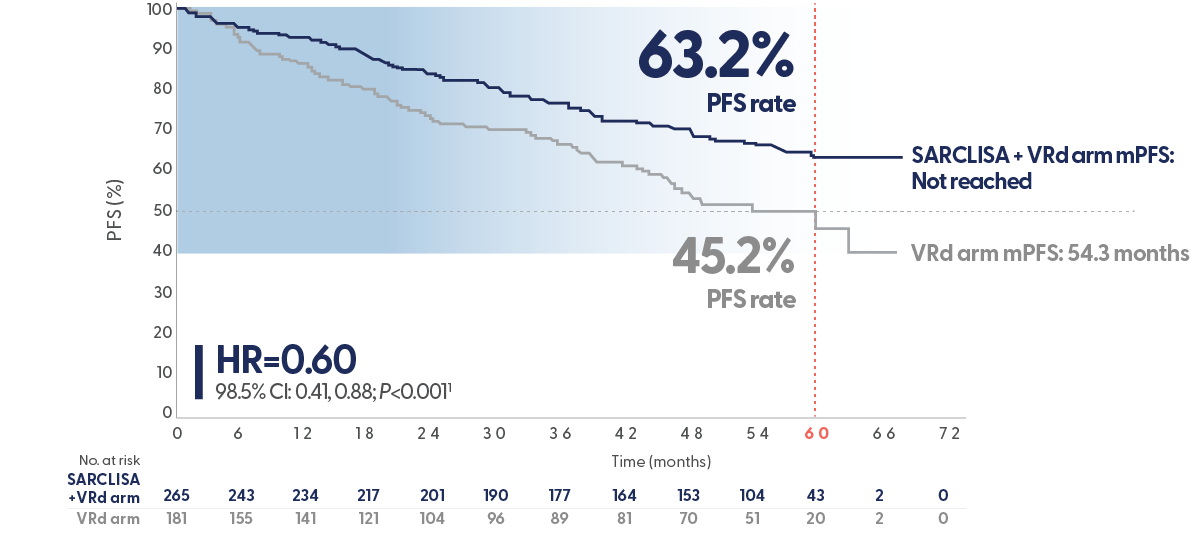
PFS results were assessed by an Independent Response Committee based on central laboratory data for M-protein and central radiologic imaging review using the IMWG criteria.1
Superior rate of complete response or better with SARCLISA + VRd vs VRd1,6
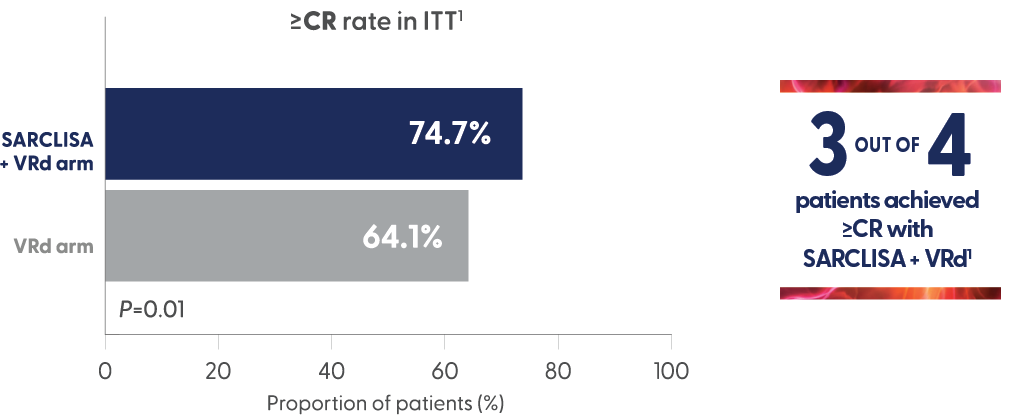
- ORR: 91.3% SARCLISA + VRd vs 92.3% VRd (for ≥CR p = 0.01)1
- ≥VGPR: 89.1% SARCLISA + VRd vs 82.9% VRd (p = >0.025)6
- The ≥VGPR was not statistically significant6
High rates of MRD negativity and sustained MRD negativity achieved in transplant ineligible NDMM patients1,6
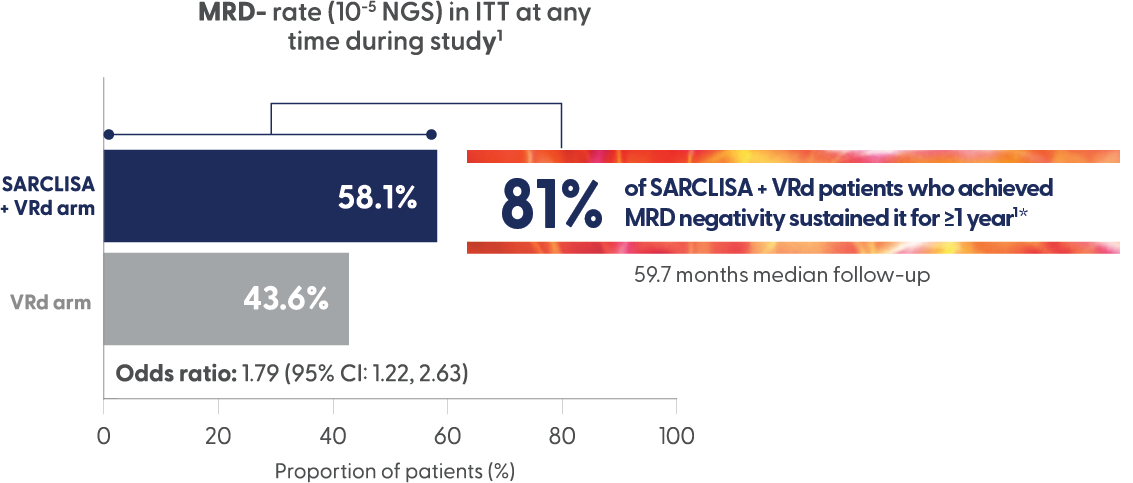
*46.8% of patients in the SARCLISA + VRd arm achieved MRD- and sustained it for ≥1 year vs 24.3% of VRd patients.1
Safety
SARCLISA + VRd showed a safety profile broadly comparable to VRd1,6
The median number of cycles received by patients in the SARCLISA + VRd group was 52 vs 29 in the VRd group. However, TEAE incidence was assessed based on duration of exposure.
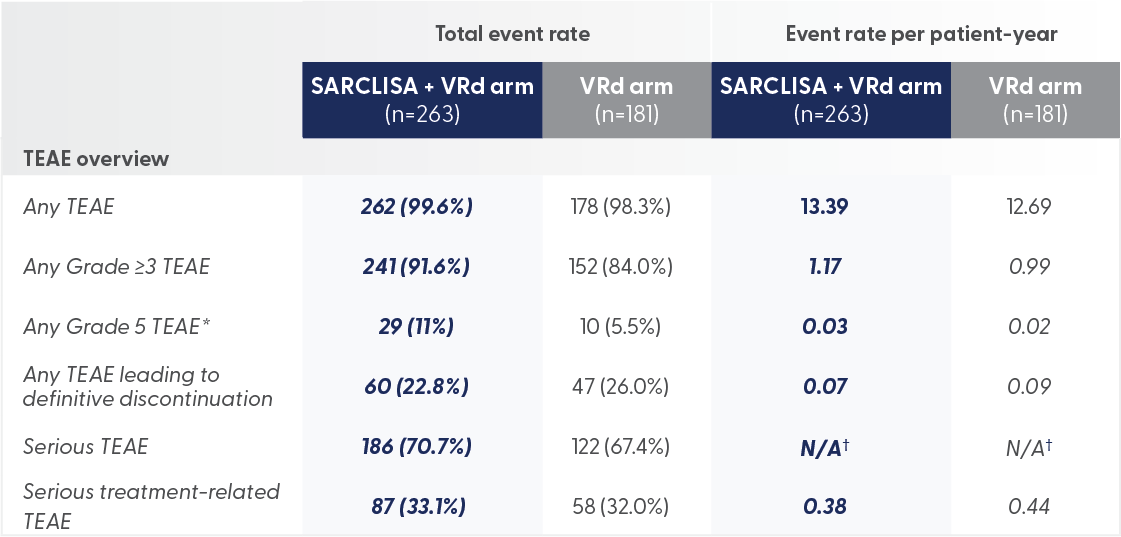
*Grade 5 TEAEs were 11% for SARCLISA + VRd vs 5.5% for VRd alone
- Grade 5 TEAEs were mainly caused by infections (7% for patients on SARCLISA + VRd, 4% for VRd), including COVID-19 (3% for patients on SARCLISA + VRd and 1% for VRd)
†Data not available
There were no new safety signals with the addition of SARCLISA to VRd1
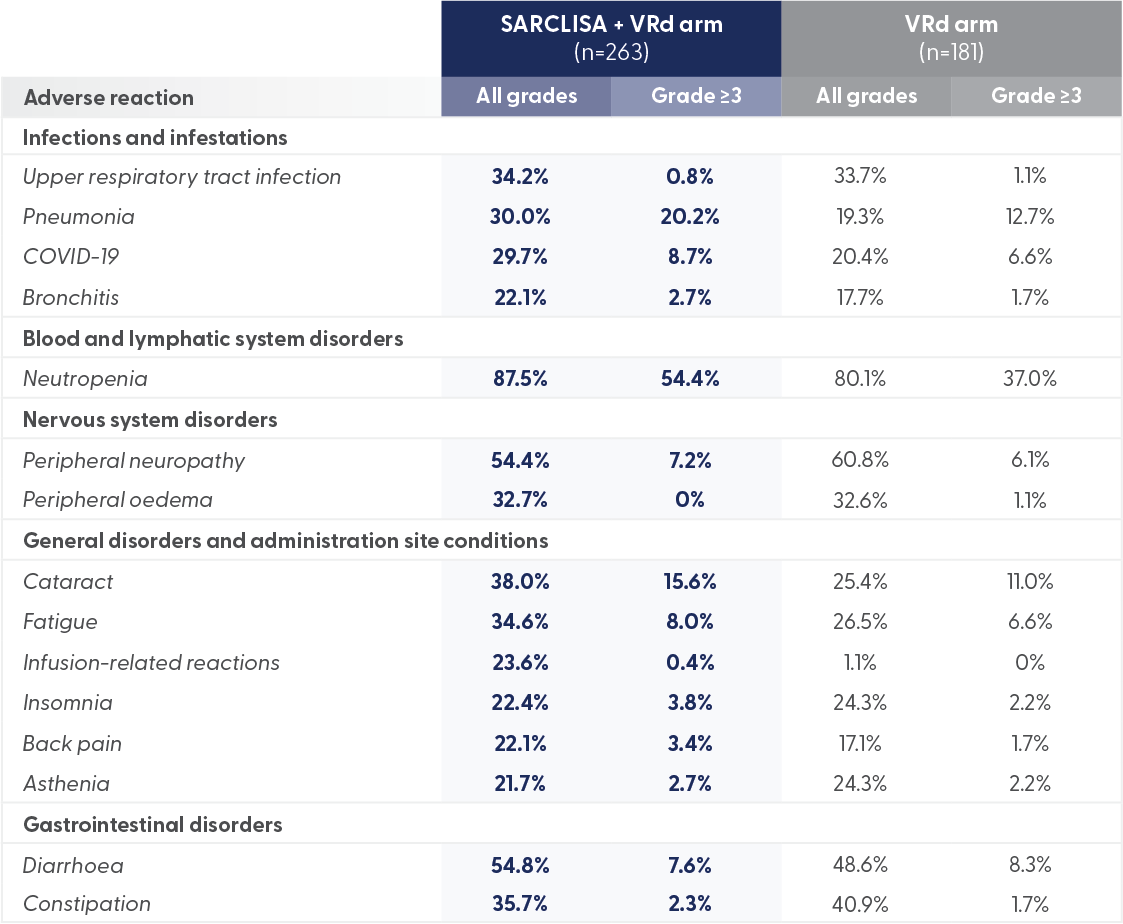
Grade 3 or 4 peripheral neuropathy was comparable in both arms.
SARCLISA + VRd showed a safety profile broadly comparable to VRd1,6
The median number of cycles received by patients in the SARCLISA + VRd group was 52 vs 29 in the VRd group. However, TEAE incidence was assessed based on duration of exposure.


*Grade 5 TEAEs were 11% for SARCLISA + VRd vs 5.5% for VRd alone
- Grade 5 TEAEs were mainly caused by infections (7% for patients on SARCLISA + VRd, 4% for VRd), including COVID-19 (3% for patients on SARCLISA + VRd and 1% for VRd)
†Data not available
There were no new safety signals with the addition of SARCLISA to VRd1

Grade 3 or 4 peripheral neuropathy was comparable in both arms.
SARCLISA + VRd (IMROZ): Infusion-related reactions
IMROZ: Infusion reactions were mostly mild to moderate and were observed in 24% of patients treated with SARCLISA + VRd.2

All patients who experienced infusion-related reactions, experienced them during the 1st infusion1

Infusion-related reactions were resolved on the same day in most patients, and all were reversible1

No delayed infusion-related reactions were observed and no mandatory post-infusion corticosteroid prophylaxis1,2

All patients who experienced infusion-related reactions, experienced them during the 1st infusion1

Infusion-related reactions were resolved on the same day in most patients, and all were reversible1

No delayed infusion-related reactions were observed and no mandatory post-infusion corticosteroid prophylaxis1,2
The most common symptoms of an infusion reaction included dyspnoea and chills. The most common severe sign and symptom was hypertension.2
Dosage
SARCLISA® + Pd (ICARIA-MM): Dosing and Administration
Recommended dosage2
10 mg/kg body weight administered as an IV infusion in combination with VRd*/250 mL fixed dosing volume.
Pre-medication† should be administered 15 to 60 minutes prior to infusion of SARCLISA.
*For other products administered with SARCLISA, refer to the respective current Summary of Product Characteristics.2
Infusion rates can increase over time, with a 75-minute infusion possible from the 3rd infusion onwards*1
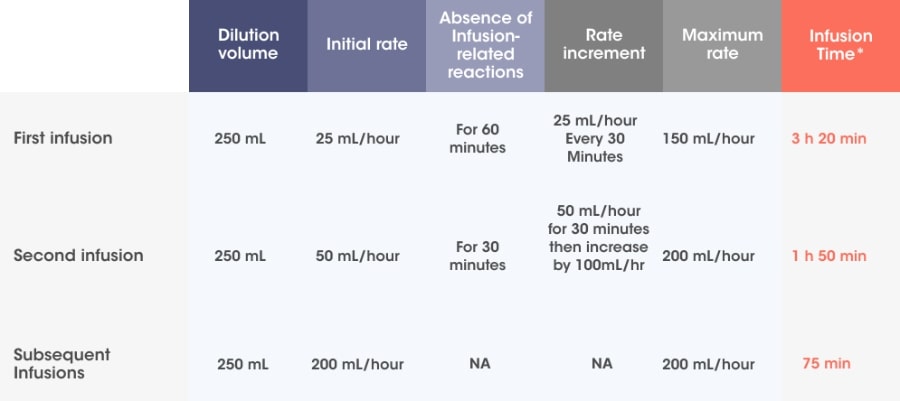
Adapted from SARCLISA® Summary of Product Characteristics.2
Incremental escalation of the infusion rate should be considered only in the absence of infusion-related reactions.2
- In patients necessitating an intervention (Grade 2, moderate infusion reactions), a temporary interruption in the infusion should be considered and additional symptomatic medicinal products can be administered. After symptom improvement to grade ≤1 (mild), SARCLISA® infusion may be resumed at half of the initial infusion rate under close monitoring and supportive care, as needed. If symptoms do not recur after 30 minutes, the infusion rate may be increased to the initial rate, and then increased incrementally.
- If symptoms do not resolve rapidly or do not improve to Grade ≤1 after interruption of SARCLISA infusion, persist or worsen despite appropriate medicinal products, or require hospitalization or are life-threatening, treatment with SARCLISA ®should be permanently discontinued and additional supportive therapy should be administered, as needed.
†Premedication should be used prior to SARCLISA infusion with the following medications to reduce the risk and severity of infusion reactions: dexamethasone 40 mg orally or IV (or 20 mg orally or IV for patients ≥75 years of age), paracetamol 650 mg to 1000 mg orally (or equivalent), H2 antagonists (ranitidine 50 mg IV or equivalent [e.g. cimetidine]), or oral proton pump inhibitors (e.g. omeprazole, esomeprazole), diphenhydramine 25 mg to 50 mg IV or orally (or equivalent [eg, cetirizine, promethazine, dexchlorpheniramine]). The IV route is preferred for at least the first 4 infusions.2
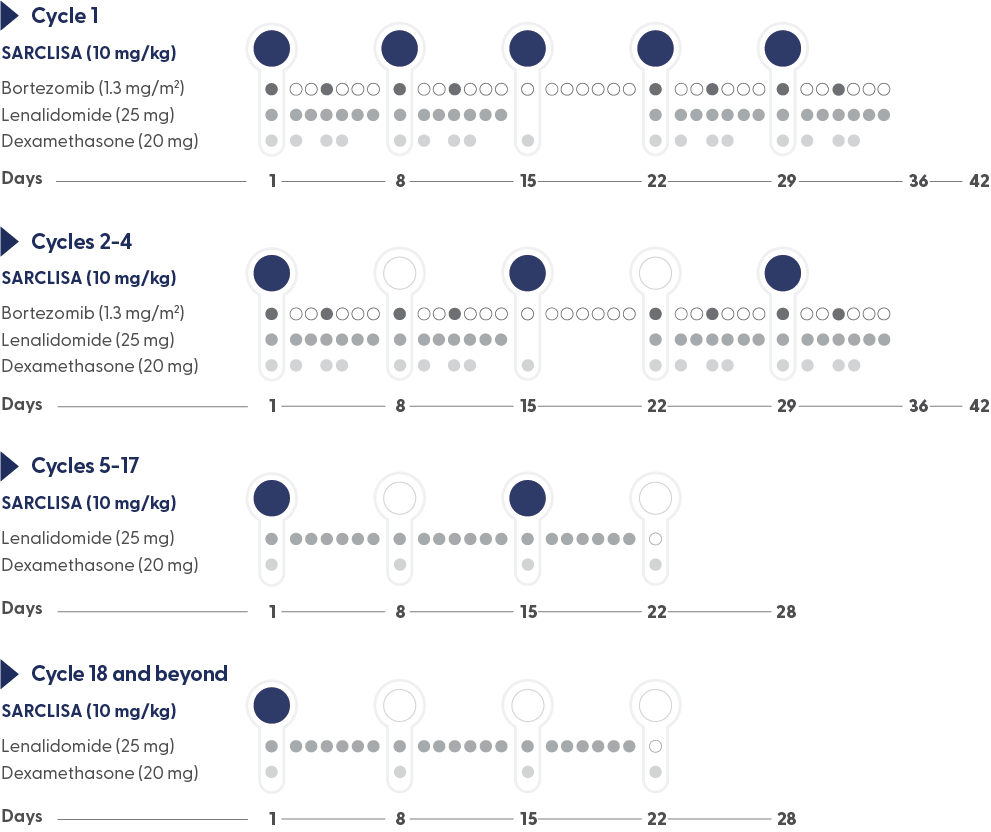
- Treatment is repeated until disease progression or unacceptable toxicity2
- For other medicinal products that are administered with SARCLISA, refer to the respective current Summary of Product Characteristics2
- If a planned dose of SARCLISA® is missed, administer the dose as soon as possible and adjust the treatment schedule accordingly, maintaining the treatment interval2
Infusion rates can increase over time, with a 75-minute infusion possible from the 3rd infusion onwards*1

Adapted from SARCLISA® Summary of Product Characteristics.2
Incremental escalation of the infusion rate should be considered only in the absence of infusion-related reactions.2
- In patients necessitating an intervention (Grade 2, moderate infusion reactions), a temporary interruption in the infusion should be considered and additional symptomatic medicinal products can be administered. After symptom improvement to grade ≤1 (mild), SARCLISA® infusion may be resumed at half of the initial infusion rate under close monitoring and supportive care, as needed. If symptoms do not recur after 30 minutes, the infusion rate may be increased to the initial rate, and then increased incrementally.
- If symptoms do not resolve rapidly or do not improve to Grade ≤1 after interruption of SARCLISA infusion, persist or worsen despite appropriate medicinal products, or require hospitalization or are life-threatening, treatment with SARCLISA ®should be permanently discontinued and additional supportive therapy should be administered, as needed.
†Premedication should be used prior to SARCLISA infusion with the following medications to reduce the risk and severity of infusion reactions: dexamethasone 40 mg orally or IV (or 20 mg orally or IV for patients ≥75 years of age), paracetamol 650 mg to 1000 mg orally (or equivalent), H2 antagonists (ranitidine 50 mg IV or equivalent [e.g. cimetidine]), or oral proton pump inhibitors (e.g. omeprazole, esomeprazole), diphenhydramine 25 mg to 50 mg IV or orally (or equivalent [eg, cetirizine, promethazine, dexchlorpheniramine]). The IV route is preferred for at least the first 4 infusions.2

- Treatment is repeated until disease progression or unacceptable toxicity2
- For other medicinal products that are administered with SARCLISA, refer to the respective current Summary of Product Characteristics2
- If a planned dose of SARCLISA® is missed, administer the dose as soon as possible and adjust the treatment schedule accordingly, maintaining the treatment interval2
MOA
SARCLISA® is an anti-CD38 mAb with a specific target epitope7,8
CD38 is a transmembrane glycoprotein that has multiple functions, including enzymatic and receptor-mediated activity.7,8
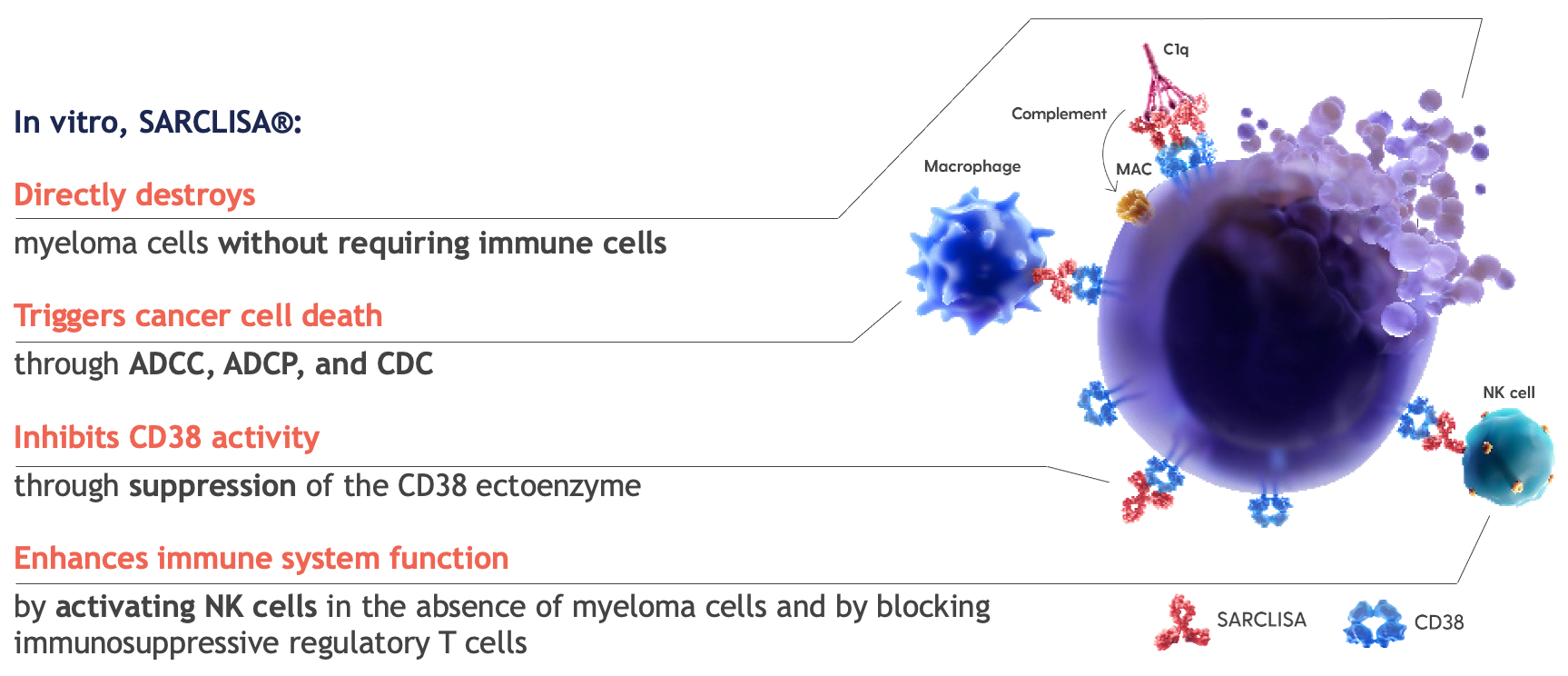
In vitro activity does not always correlate to clinical efficacy.
Multimodal actions:7,8

Inhibits CD38 enzymatic activity through suppression of the CD38 ectoenzyme

Triggers myeloma cell death through tumour cell targeting, including ADCC, ADCP, and CDC

Enhances immune cell function through NK cell activation and a decrease in immuno-suppressors

Directly destroys myeloma cells through apoptosis, without the need for crosslinking
ASCT: autologous stem cell transplant, CI: confidence interval, CR: complete response, ECOG: Eastern Cooperative Oncology Group, eGFR: estimated glomerular filtration rate, HR: hazard ratio, IMWG: International Myeloma Working Group, mAb: monoclonal antibody, MOA: mode of action, mPFS: median progression-free survival, MRD-: minimal residual disease negativity, NDMM: newly diagnosed multiple myeloma, ORR: overall response rate, OS: overall survival, PFS: progression-free survival, TEAE: treatment emergent adverse event, VGPR: very good partial response, VRd: bortezomib, lenalidomide, dexamethasone
References
- Facon T, Dimopoulos MA, Leleu XP, et al. Isatuximab, bortezomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. Published online June 3, 2024. doi:10.1056/NEJMoa2400712
- SARCLISA summary of product characteristics.
- Killmurray C. Daratumumab shifts approach in newly diagnosed multiple myeloma. Peers Perspectives Oncol. 2023;1(3):91-94.
- REVLIMID summary of product characteristics.
- VELCADE summary of product characteristics.
- Supplement to: Facon T, Dimopoulos MA, Leleu XP, et al. Isatuximab, bortezomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. Published online June 3, 2024. doi:10.1056/NEJMoa2400712
- Attal M et al. Lancet. 2019;394(10214):2096-2107. doi:10.1016/S0140-6736(19)32556-5.
- Feng X et al. Clin Cancer Res. 2017;23(15):4290-4300.
MAT-XU-2502442 (v1.0) Date of preparation: August 2025