
Toujeo is indicated for the treatment of diabetes mellitus in adults, adolescents and children from the age of 6 years.1
For patients with type 1 or 2 diabetes who need basal insulin, Toujeo® helps provide a balance between effective HbA1c control and risk of hypoglycaemia.2-7
Toujeo features

Toujeo® can give your patients sustained glycaemic control when administered once daily in the morning or evening1
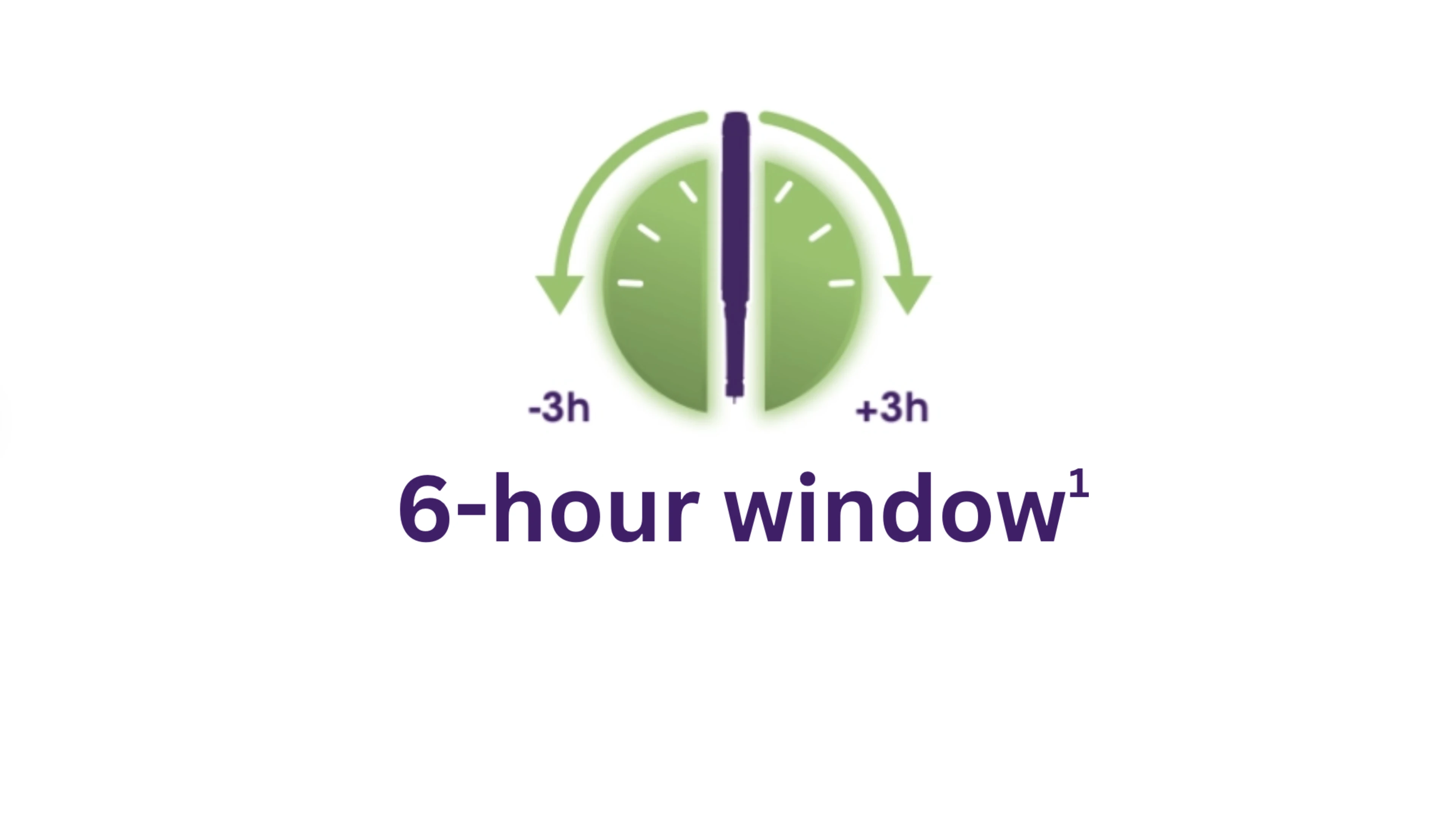
Toujeo® can give your patients flexibility around their injection time to fit in with their daily lives when needed1

The pen that was rated by 97.5% of adult patients as easy to use8

Toujeo® can give your patients sustained glycaemic control when administered once daily in the morning or evening1

Toujeo® can give your patients flexibility around their injection time to fit in with their daily lives when needed1

The pen that was rated by 97.5% of adult patients as easy to use8
Starting and switching patients to a basal insulin
Starting Toujeo:1
- Once daily at any time, preferably at the same time each day. When needed, patients can administer within a 3-hour window of their usual administration.
- Must be combined with short/rapid-acting insulin in Type 1 diabetes.
- Can be used with other anti-hyperglycaemics in Type 2 diabetes.
Switching to Toujeo® from*:1
- From once-daily basal insulins: Unit-to-unit conversion based on previous dose**.
- From twice-daily basal insulins: Start with 80% of total daily basal insulin dose.
Close metabolic monitoring is recommended during the switch and in the initial weeks thereafter.
Patients with high insulin doses because of antibodies to human insulin may experience an improved insulin response with Toujeo.
*When switching basal insulin to Toujeo®, dose reduction of basal and bolus insulin needs to be considered on an individual basis to avoid hypoglycaemia.
**When switching from insulin glargine 100 units/ml to Toujeo, this can be done on a unit-to-unit basis, but a higher Toujeo dose (approximately 10-18%) may be needed to achieve target ranges for plasma glucose levels.
How to order Toujeo
|
Presentation |
PIP Code |
Toujeo NHS list price |
|
Toujeo® SoloSTAR® |
3981354 |
£32.14 |
|
Toujeo® DoubleSTAR® |
4104782 |
£64.27 |
Toujeo DoubleStar prefilled pen is recommended for patients requiring at least 20 units per day.
There are two channels through which you can order Toujeo.
Via AAH:
- Community Pharmacy - 0344 561 8899
- Dispensing Doctors - 0344 561 8899
- Hospitals - 0344 561 6699
Via Phoenix:
- 01928 750 500 / 01928 750 750
If you are having any issues in getting stock of Toujeo from the above wholesalers, in that instance please contact Sanofi Customer Service on gb-customerservices@sanofi.com for further support.
More about diabetes

.jpg)
Hypoglycaemia is a very common side effect of Toujeo. Hypoglycaemia, in general the most frequent adverse reaction of insulin therapy, may occur if the insulin dose is too high in relation to the insulin requirement. Lipohypertrophy and injection site reactions are common side effects.1 For further information on the safety profile of Toujeo, please consult the full Summary of Product Characteristics
For further information on the safety profile of Toujeo® please consult the Summary of Product Characteristics.
-
Toujeo® Summary of Product Characteristics. Available at: https://www.medicines.org.uk/emc/product/6938/smpc. [Accessed August 2025].
-
Danne T, Matsuhisa M et al. Lower risk of severe hypoglycaemia with insulin glargine 300 U/mL versus glargine 100 U/mL in participants with type 1 diabetes: a meta-analysis of 6-month phase 3 clinical trials. Diabetes Obes Metab 2020;22(10):1880-1885
-
Ritzel R, Roussel R et al. Patient-level meta-analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus glargine 100 U/ml in people with type 2 diabetes. Diabetes Obes Metab 2015;17(9):859-867
-
Ritzel R, Roussel R et al. Better glycaemic control and less hypoglycaemia with insulin glargine 300 U/mL vs glargine 100 U/mL: 1-year patient-level meta-analysis of the EDITION clinical studies in people with type 2 diabetes. Diabetes Obes Metab 2018;20(3):541-548
-
Battelino T, Bosnyak Z et al. InRange: comparison of the second-generation basal insulin analogues glargine 300 u/ml and degludec 100 u/ml in persons with type 1 diabetes using continuous glucose monitoring-study design. Diabetes Ther 2020;11(4):1017-1027
-
Battelino T, Danne T et al. Continuous glucose monitoring-based time-in-range using insulin glargine 300 units/ml versus insulin degludec 100 units/ml in type 1 diabetes: the head-to-head randomised controlled InRange trial. Diabetes Obes Metab 2023;25(2):545-555
-
Rosenstock J, Cheng A et al. More similarities than differences testing insulin glargine 300 units/ml versus insulin degludec 100 units/ml in insulin-naive type 2 diabetes: the randomised head-to-head BRIGHT trial. Diabetes Care 2018;41(10):2147-2154
-
Pohlmeier H, et al. J Diabetes Sci Technol.2017:11;263-269
HbA1c - Haemoglobin A1c
MAT-XU-2302426 (v15.0) Date of Preparation: August 2025
