- Article
- Source: Campus Sanofi
- 10 Oct 2025
ROCKreal: Real-World Clinical Insights

ROCKreal study1
ROCKreal shows REZUROCK is an effective option for improving outcomes over BAT for patients with cGVHD.
Study design: ROCKreal was designed to emulate a phase 3 RCT via casual inference methodology. Consisted of non-interventional chart review from 8 transplant centres in the United States.
Primary end point: Overall response rate (ORR) at 6 months.
Key Findings:
Efficacy and safety findings are consistent with ROCKstar trial.
Patients treated with belumosudil had improved outcomes vs BAT, regardless of LOT highlighting its effectiveness.






BAT, best available therapy; RCT, randomised control trial; cGVHD, chronic graft-vs-host-disease; FFS, failure-free survival; LOT, lines of therapy; ORR, overall response rate.
Study Summary

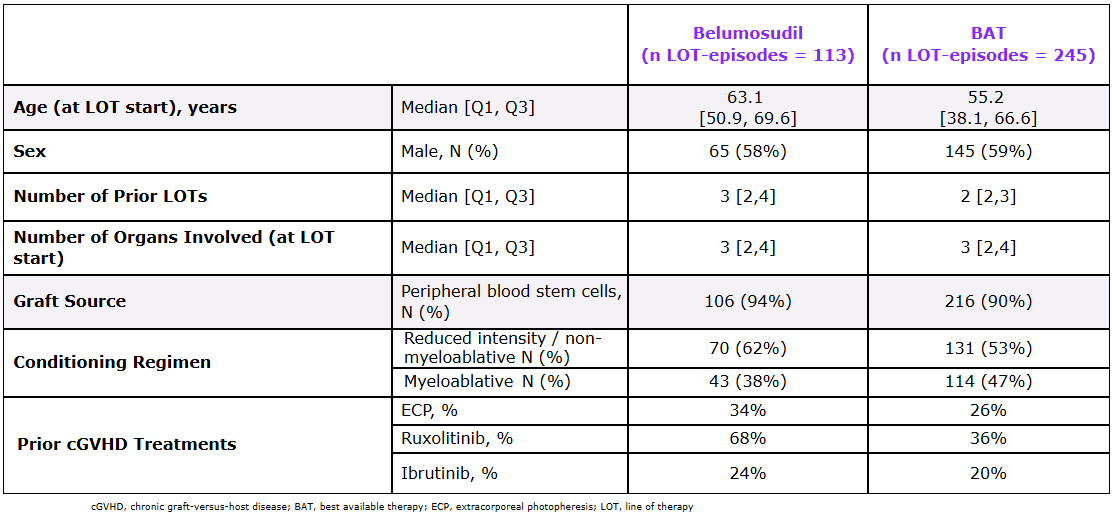
Patient Characteristics
Primary Endpoint: Overall Response Rate at 6 Months
44.2% ratio improvement (11.9% absolute improvement) in the primary endpoint with REZUROCK vs BAT
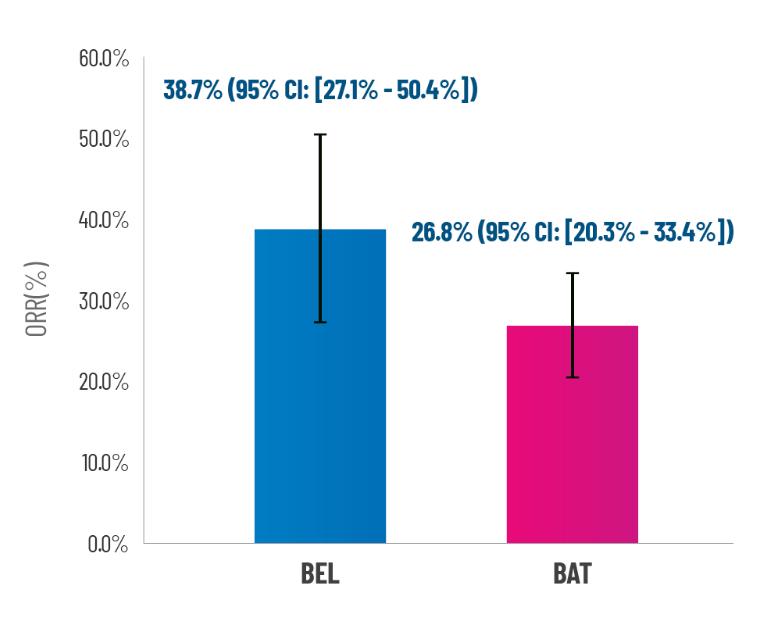
Patients were classified as responders (OR=1) if they had a complete or partial response or 50% corticosteroid (CS) dose reduction without disease progression.
Failure was recorded for mixed responses, disease progression, death, treatment switch, or relapse.
BEL, belumosudil, BAT, best available therapy, ORR, overall response rate, CI, confidence interval
Secondary Endpoint: Failure-Free Survival at 1 year2
13.5% improvement in adjusted FFS at 1 year when treated with REZUROCK vs BAT
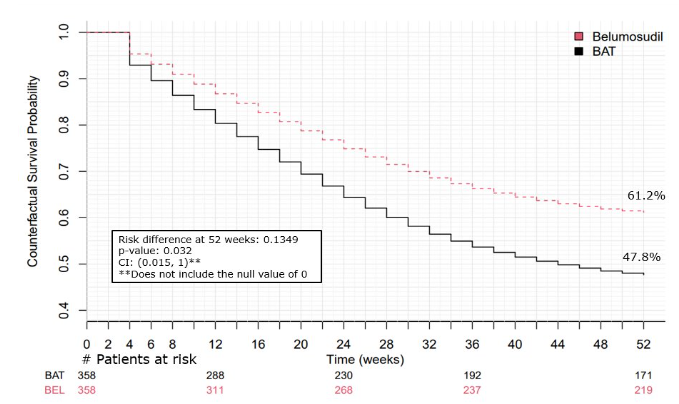
The difference in 1-year cumulative incidence of failure was -0.13 (95% CI:[-1, -0.015], p-value: 0.032). This indicates a statistically significant reduction in failure events and an increase in 1-year FFS with REZUROCK compared to BAT.
.jpg)
1. Hall K, et al. Presented at EBMT 2025 (OS11-03)
2. Hall K, et al. Blood Advances, 2025. doi: 10.1182/bloodadvances.2025015832
MAT-XU-2504746 (v1.0) Date of Preparation: January 2026