.png)
ALPROLIX prophylaxis offers proven protection* at dosing intervals to fit your patients’ needs

7-Day interval
For dosing on the same day every week1
- The recommended starting dose is 50 IU/kg for adults/adolescents (aged ≥12 years) and 60 IU/kg for children (aged ≤11 years)
- A 7-day interval is the most common EHL prophylaxis regimen in hemophilia B†

10-Day interval
For less frequent dosing1
- Another recommended starting dose is 100 IU/kg for adults and adolescents

14-Day interval
Potential for ≥14-day dosing for fewer infusions2,3
- In the B-LONG and B-YOND trials, some adult and adolescent patients extended their dosing interval to ≥14 days
For patients on ALPROLIX looking to extend their dosing interval beyond their initial starting interval, adjust the dose based on individual patient response
Dose and duration of treatment depend on the severity of the factor IX deficiency, the location and extent of bleeding, the individual patient’s PK profile, and/or the patient’s clinical condition
Clinical trial information
B-LONG was a phase 3 open-label study that investigated the safety and efficacy of ALPROLIX in 123 previously treated adult and adolescent patients aged ≥12 years with severe hemophilia B. The study included a fixed-interval (weekly) arm (n=63), a fixed-dose (interval-adjusted) arm (n=29), an episodic (on-demand) arm (n=27), and a surgical arm (n=12).1
B-YOND was an open-label extension trial that studied the long-term safety and efficacy of ALPROLIX over 5 years in 120 adult, adolescent, and pediatric patients previously treated in Kids B-LONG or B-LONG. The study included a fixed-interval arm (n=74), a fixed-dose arm (n=36), a modified prophylaxis arm (n=17), and an episodic (on-demand) arm (n=15).4
* ALPROLIX has been proven to help patients prevent bleeding episodes using a prophylaxis regimen.1
† Data are from an online survey conducted by Sanofi in April 2019 with 359 adult patients and caregivers to provide insights into the US hemophilia A and hemophilia B markets. Ninety respondents were adults with hemophilia B and 60 respondents were caregivers of a patient with hemophilia B.
EHL=extended half-life; PK=pharmacokinetic; SHL=standard half-life.

Pediatric Patients Dosing
.png)
Adults and Adolescents Dosing

Watch Dr De Angulo discuss the protection
Watch Dr de angulo discuss the protection,* experience, and flexibility of ALPROLIX across treatment scenarios
*ALPROLIX has been proven to help patients prevent bleeding episodes using a prophylaxis regimen.1
Pediatric Patients Dosing
With ALPROLIX, children can start on a treatment that can grow with them, as their needs may change over time1
For children aged <12 years, the recommended prophylaxis starting regimen is 60 IU/kg once weekly, with adjustments made based on patient response1
- More frequent or higher doses may be needed in children aged <12 years, and especially in children aged <6 years
- On average, 1 IU of ALPROLIX per kg of body weight increases the level of circulating factor IX by approximately 1% in children aged ≥6 years and 0.6% in children aged <6 years

~93% of pediatric patients maintained or extended their dosing interval through the B-YOND extension trial4
- In B-YOND (n=27), 21 patients maintained and 4 patients extended their dosing interval
Adults and Adolescents Dosing
ALPROLIX offers flexibility of prophylaxis dosing intervals to fit your patients’ needs
For patients who want a regimen on the same day every week, initiate treatment at 50 IU/kg every 7 days1
For fewer infusions, ALPROLIX offers a second prophylaxis starting dose1
- ALPROLIX can be initiated at 100 IU/kg every 10 days
- To extend the dosing interval beyond a patient’s initial starting interval, adjust the dose based on individual patient response
- Dose and duration of treatment depend on the severity of the factor IX deficiency, the location and extent of bleeding, the individual patient’s pharmacokinetic profile, and/or the patient’s clinical condition

85% of adult and adolescent patients maintained or extended their dosing interval through the B-YOND extension trial4
- In B-YOND (n=71), 56 patients maintained and 4 patients extended their dosing interval
.png)
≥14-Day dosing for patients who want extended dosing, ALPROLIX offers the potential for ≥14-day dosing
- More than half of patients (54%) in the interval-adjusted arm (n=26) extended their dosing interval to ≥14 days during the last 3 months of B-LONG2
Average dosing interval for interval-adjusted arm2
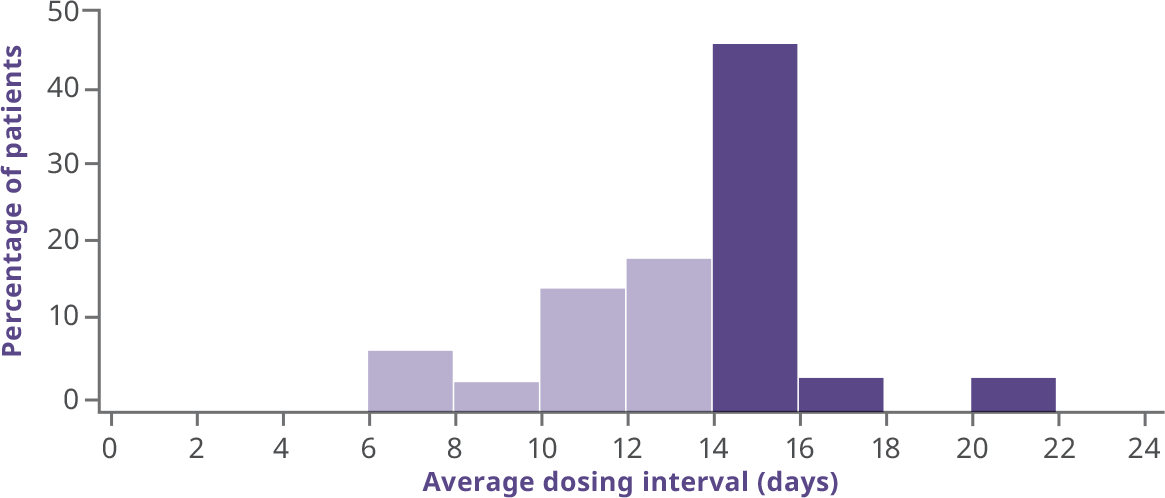
- Patients initiated treatment at 100 IU/kg every 10 days; the majority were well controlled and experienced zero bleeds before extending their interval3
- Overall median dosing interval in B-LONG was 12.5 days (10.4-13.4)1
- Additional patients were able to extend their dosing interval during the B-YOND extension trial3
Post hoc analysis: ALPROLIX prophylaxis efficacy data at ≥14-day interval (n=22)3:
- Median overall ABR:
1.6 (0.6-2.7)
- Median AsBR:
0.7 (0.3-1.1)
- Median joint ABR:
1.0 (0.3-1.6)
- Data are reported from a post hoc analysis of 22 adult and adolescent patients (aged ≥12 years) who received ALPROLIX prophylaxis with a dosing interval of ≥14 days at any time during B-LONG or B-YOND, up until the time of the second interim analysis
- The analysis was not powered to show statistical significance. There was a small sample size, no control group, and potential for interobserver variability
- Patients in the Kids B-LONG study were not included in this post hoc analysis because the study design did not allow for dosing intervals longer than 1 week
In the interval-adjusted arm of B-LONG (n=29)3:
- Median overall ABR:
1.38 (0-3.43)
- Median AsBR:
0.88 (0-2.3)
- Median joint ABR:
0.36 (0-3.24)
The median duration of the exposure to ALPROLIX at a ≥14-day dosing interval was 3.4 years, as reported in the post hoc analysis3
Protection,* Experience, and Dosing:
a discussion with Dr. De Angulo
*ALPROLIX has been proven to help patients prevent bleeding episodes using a prophylaxis regimen.1
Dr Guillermo De Angulo shares his clinical experience with ALPROLIX.
Hear about how he integrates ALPROLIX into his practice and how he evaluates his patients’ ongoing treatment.
INDICATION:
ABR=annualized bleed rate; AsBR=annualized spontaneous bleed rate.
References: 1. ALPROLIX [package insert]. Waltham, MA: Bioverativ Therapeutics Inc. 2. Powell JS, Pasi KJ, Ragni MV, et al. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N Engl J Med. 2013;369(24):2313-2323. 3. Shapiro AD, Pasi KJ, Ozelo MC, et al. Extending recombinant factor IX Fc fusion protein dosing interval to 14 or more days in patients with hemophilia B. Res Pract Thromb Haemost. 2018;3(1):109-113. 4. Pasi KJ, Fischer K, Ragni M, et al. Long-term safety and sustained efficacy for up to 5 years of treatment with recombinant factor IX Fc fusion protein in subjects with haemophilia B: results from the B-YOND extension study. Haemophilia. 2020;26(6):e262-e271. 5. Idelvion® [package insert]. Marburg, Germany: CSL Behring GmbH; 2021. 6. Rebinyn® [package insert]. Plainsboro, NJ: Novo Nordisk Inc; 2022. 7. AlphaNine® SD [package insert]. Los Angeles, CA: Grifols Biologicals LLC; 2021. 8. BeneFIX® [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc., a subsidiary of Pfizer Inc.; 2021. 9. Ixinity® [package insert]. Seattle, WA: Aptevo BioTherapeutics LLC; 2022. 10. Rixubis® [package insert]. Lexington, MA: Baxalta US Inc.; 2020.